Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice
- PMID: 10637275
- PMCID: PMC2195765
- DOI: 10.1084/jem.191.2.313
Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice
Abstract
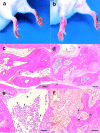
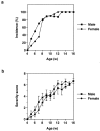
Interleukin (IL)-1 is a proinflammatory cytokine that plays important roles in inflammation, host defense, and the neuro-immuno-endocrine network. IL-1 receptor antagonist (ra) is an endogenous inhibitor of IL-1 and is supposed to regulate IL-1 activity. However, its pathophysiological roles in a body remain largely unknown. To elucidate the roles of IL-1ra, IL-1ra-deficient mice were produced by gene targeting, and pathology was analyzed on different genetic backgrounds. We found that all of the mice on a BALB/cA background, but not those on a C57BL/6J background, spontaneously developed chronic inflammatory polyarthropathy. Histopathology showed marked synovial and periarticular inflammation, with articular erosion caused by invasion of granulation tissues closely resembling that of rheumatoid arthritis in humans. Moreover, elevated levels of antibodies against immunoglobulins, type II collagen, and double-stranded DNA were detected in these mice, suggesting development of autoimmunity. Proinflammatory cytokines such as IL-1beta, IL-6, and tumor necrosis factor alpha were overexpressed in the joints, indicating regulatory roles of IL-1ra in the cytokine network. We thus show that IL-1ra gene deficiency causes autoimmunity and joint-specific inflammation and suggest that IL-1ra is important in maintaining homeostasis of the immune system. Possible involvement of IL-1ra gene deficiency in RA will be discussed.
Figures



References
-
- Firestein, G.S., and N.J. Zvaifler. 1992. Rheumatoid arthritis: a disease of disordered immunity. In Inflammation: Basic Principles and Clinical Correlates, 2nd ed. Raven Press, Ltd., New York. 959–975.
-
- Feldmann M., Brennan F.M., Maini R.N. Role of cytokines in rheumatoid arthritis. Annu. Rev. Immunol. 1996;14:397–440. - PubMed
-
- Marinova-Mutafchieva L., Williams R.O., Mason L.J., Mauri C., Feldmann M., Maini R.N. Dynamics of proinflammatory cytokine expression in the joints of mice with collagen-induced arthritis (CIA) Clin. Exp. Immunol. 1997;107:507–512. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

