Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand
- PMID: 10637280
- PMCID: PMC2195745
- DOI: 10.1084/jem.191.2.365
Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand
Abstract
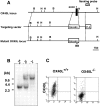
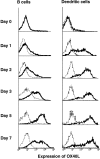
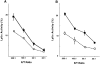
OX40 expressed on activated T cells is known to be an important costimulatory molecule on T cell activation in vitro. However, the in vivo functional significance of the interaction between OX40 and its ligand, OX40L, is still unclear. To investigate the role of OX40L during in vivo immune responses, we generated OX40L-deficient mice and a blocking anti-OX40L monoclonal antibody, MGP34. OX40L expression was demonstrated on splenic B cells after CD40 and anti-immunoglobulin (Ig)M stimulation, while only CD40 ligation was capable of inducing OX40L on dendritic cells. OX40L-deficient and MGP34-treated mice engendered apparent suppression of the recall reaction of T cells primed with both protein antigens and alloantigens and a significant reduction in keyhole limpet hemocyanin-specific IgG production. The impaired T cell priming was also accompanied by a concomitant reduction of both T helper type 1 (Th1) and Th2 cytokines. Furthermore, antigen-presenting cells (APCs) derived from the mutant mice revealed an impaired intrinsic APC function, demonstrating the importance of OX40L in both the priming and effector phases of T cell activation. Collectively, these results provide convincing evidence that OX40L, expressed on APCs, plays a critical role in antigen-specific T cell responses in vivo.
Figures



References
-
- Paterson D.J., Jefferies W.A., Green J.R., Brandon M.R., Corthesy P., Puklavec M., Williams A.F. Antigens of activated rat T lymphocytes including a molecule of 50,000 M r detected only on CD4 positive T blasts. Mol. Immunol. 1987;24:1281–1290. - PubMed
-
- Al-Shamkhani A., Birkeland M.L., Puklavec M., Brown M.H., James W., Barclay A.N. OX40 is differentially expressed on activated rat and mouse T cells and is the sole receptor for the OX40 ligand. Eur. J. Immunol. 1996;26:1695–1699. - PubMed
-
- Calderhead D.M., Buhlmann J.E., Van der Eetwegh A.J.M., Claasen E., Noelle R.J., Fell H.P. Cloning of mouse Ox40a T cell activation marker that may mediate T-B interactions. J. Immunol. 1993;151:5261–5271. - PubMed
-
- Baum P.R., Gayle R.B., Ramsdell F., Srinivasan S., Sorensen R.A., Watson M.L., Seldin M.F., Baker E., Sutherland G.R., Clifford K.N. Molecular characterization of murine and human OX-40/OX-40 ligand systemsidentification of a human OX-40 ligand as the HTLV-1-regulated protein gp34. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:3992–4001. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

