The N terminus of the transmembrane protein BP180 interacts with the N-terminal domain of BP230, thereby mediating keratin cytoskeleton anchorage to the cell surface at the site of the hemidesmosome
- PMID: 10637308
- PMCID: PMC14774
- DOI: 10.1091/mbc.11.1.277
The N terminus of the transmembrane protein BP180 interacts with the N-terminal domain of BP230, thereby mediating keratin cytoskeleton anchorage to the cell surface at the site of the hemidesmosome
Abstract
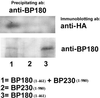
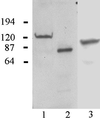
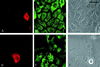
In epidermal cells, the keratin cytoskeleton interacts with the elements in the basement membrane via a multimolecular junction called the hemidesmosome. A major component of the hemidesmosome plaque is the 230-kDa bullous pemphigoid autoantigen (BP230/BPAG1), which connects directly to the keratin-containing intermediate filaments of the cytoskeleton via its C terminus. A second bullous pemphigoid antigen of 180 kDa (BP180/BPAG2) is a type II transmembrane component of the hemidesmosome. Using yeast two-hybrid technology and recombinant proteins, we show that an N-terminal fragment of BP230 can bind directly to an N-terminal fragment of BP180. We have also explored the consequences of expression of the BP230 N terminus in 804G cells that assemble hemidesmosomes in vitro. Unexpectedly, this fragment disrupts the distribution of BP180 in transfected cells but has no apparent impact on the organization of endogenous BP230 and alpha6beta4 integrin. We propose that the BP230 N terminus competes with endogenous BP230 protein for BP180 binding and inhibits incorporation of BP180 into the cell surface at the site of the hemidesmosome. These data provide new insight into those interactions of the molecules of the hemidesmosome that are necessary for its function in integrating epithelial and connective tissue types.
Figures

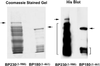


References
-
- Aho S, Uitto J. Direct interaction between the intracellular domains of bullous pemphigoid antigen 2 (BP180) and β4 integrin, hemidesmosomal components of basal keratinocytes. Biochem Biophys Res Commun. 1998;243:694–699. - PubMed
-
- Borradori L, Chavanas S, Schaapveld RQJ, Gagnoux-Placacios L, Calafat J, Meneguzzi G, Sonnenberg A. Role of the bullous pemphigoid antigen 180 (BP180) in the assembly of hemidesmosomes and cell adhesion: reexpression of BP180 in generalized atrophic benign epidermolysis bullosa keratinocytes. Exp Cell Res. 1998;239:463–476. - PubMed
-
- Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: more than simple adhesion complexes. J Invest Dermatol. 1999;112:411–418. - PubMed
-
- Chavanas S, Gache Y, Tadini G, Pulkkinen L, Uitto J, Ortonne JP, Meneguzzi G. A homozygous in-frame deletion in the collagenous domain of bullous pemphigoid antigen BP180 (type XVII collagen) causes generalized atrophic benign epidermolysis bullosa. J Invest Dermatol. 1997;109:74–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

