The 4'lysine in the putative channel lining domain affects desensitization but not the single-channel conductance of recombinant homomeric 5-HT3A receptors
- PMID: 10639097
- PMCID: PMC2269757
- DOI: 10.1111/j.1469-7793.2000.00187.x
The 4'lysine in the putative channel lining domain affects desensitization but not the single-channel conductance of recombinant homomeric 5-HT3A receptors
Abstract
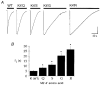
The 5-HT3 receptor is a transmitter-gated ion channel of the Cys-loop superfamily. Uniquely, 5-HT3 receptor subunits (5-HT3A and 5-HT3B) possess a positively charged lysine residue within the putative channel lining M2 domain (4' position). Using whole cell recording techniques, we examined the role of this residue in receptor function using wild-type (WT) and mutant 5-HT3A receptor subunits of murine origin transiently expressed in human embryonic kidney (HEK 293) cells. WT 5-HT3A receptors mediated rapidly activating currents in response to 5-HT (10-90 % rise time, 103 ms; EC50, 2.34 microM; Hill coefficient, nH, 2.87). The currents rectified inwardly, reversed in sign at a potential of -9 mV and desensitized in the continuous presence of agonist (half-time of desensitization, t(1/2), 2.13 s). 5-HT3A receptor subunits in which the 4'lysine was mutated to arginine, glutamine, serine or glycine formed functional receptors. 5-HT EC50 values were approximately 2-fold lower than for WT 5-HT3A receptors, but Hill coefficients, kinetics of current activation, rectification, and reversal potentials were unaltered. Each of the mutants desensitized more slowly than the WT 5-HT3A receptor, with the arginine and glycine mutations exhibiting the greatest effect (5-fold reduction). The rank order of effect was arginine > glycine > serine > glutamine. The single-channel conductance of the WT 5-HT3A receptor, as assessed by fluctuation analysis of macroscopic currents, was 390 fS. A similar value was obtained for the 4'lysine mutant receptors. Thus it appears unlikely that 4'lysine is exposed to the channel lumen. Mutation of residues immediately adjacent to 4'lysine to glutamate or lysine resulted in lack of receptor expression or function. We conclude that 4'lysine does not form part of the channel lining, but may play an important role in 5-HT3 receptor desensitization.
Figures


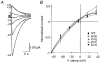


References
-
- Akabas MH, Kaufmann C, Archdeacon P, Karlin A. Identification of acetylcholine receptor channel-lining residues in the entire M2 segment of the α-subunit. Neuron. 1994;13:919–927. - PubMed
-
- Barnard EA. The transmitter-gated channels: a range of receptor types and structures. Trends in Pharmacological Sciences. 1996;17:305–309. - PubMed
-
- Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. Journal of Membrane Biology. 1991;121:101–117. - PubMed
-
- Belelli D, Balcarek JM, Hope AG, Peters JA, Lambert JJ, Blackburn TP. Cloning and functional expression of a human 5-hydroxytryptamine type 3AS receptor subunit. Molecular Pharmacology. 1995;48:1054–1062. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

