NifS-directed assembly of a transient [2Fe-2S] cluster within the NifU protein
- PMID: 10639125
- PMCID: PMC15376
- DOI: 10.1073/pnas.97.2.599
NifS-directed assembly of a transient [2Fe-2S] cluster within the NifU protein
Abstract
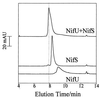
The NifS and NifU proteins from Azotobacter vinelandii are required for the full activation of nitrogenase. NifS is a homodimeric cysteine desulfurase that supplies the inorganic sulfide necessary for formation of the Fe-S clusters contained within the nitrogenase component proteins. NifU has been suggested to complement NifS either by mobilizing the Fe necessary for nitrogenase Fe-S cluster formation or by providing an intermediate Fe-S cluster assembly site. As isolated, the homodimeric NifU protein contains one [2Fe-2S](2+, +) cluster per subunit, which is referred to as the permanent cluster. In this report, we show that NifU is able to interact with NifS and that a second, transient [2Fe-2S] cluster can be assembled within NifU in vitro when incubated in the presence of ferric ion, L-cysteine, and catalytic amounts of NifS. Approximately one transient [2Fe-2S] cluster is assembled per homodimer. The transient [2Fe-2S] cluster species is labile and rapidly released on reduction. We propose that transient [2Fe-2S] cluster units are formed on NifU and then released to supply the inorganic iron and sulfur necessary for maturation of the nitrogenase component proteins. The role of the permanent [2Fe-2S] clusters contained within NifU is not yet known, but they could have a redox function involving either the formation or release of transient [2Fe-2S] cluster units assembled on NifU. Because homologs to both NifU and NifS, respectively designated IscU and IscS, are found in non-nitrogen fixing organisms, it is possible that the function of NifU proposed here could represent a general mechanism for the maturation of Fe-S cluster-containing proteins.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

