Extracellular protein kinase A as a cancer biomarker: its expression by tumor cells and reversal by a myristate-lacking Calpha and RIIbeta subunit overexpression
- PMID: 10639166
- PMCID: PMC15417
- DOI: 10.1073/pnas.97.2.835
Extracellular protein kinase A as a cancer biomarker: its expression by tumor cells and reversal by a myristate-lacking Calpha and RIIbeta subunit overexpression
Abstract
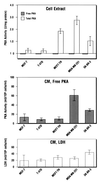
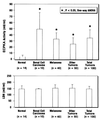
Overexpression of cAMP-dependent protein kinase (PKA) type I isozyme is associated with cell proliferation and neoplastic transformation. The presence of PKA on the external surface of LS-174T human colon carcinoma cells has been shown. Here, we show that cancer cells of various cell types excrete PKA into the conditioned medium. This extracellular PKA (ECPKA) is present in active, free catalytic subunit (C subunit) form, and its activity is specifically inhibited by PKA inhibitory protein, PKI. Overexpression of the Calpha or RIalpha subunit gene of PKA in an expression vector, which up-regulates intracellular PKA type I, markedly up-regulates ECPKA expression. In contrast, overexpression of the RIIbeta subunit, which eliminates PKA type I, up-regulates PKA type II, and reverts the transformed phenotype, down-regulates ECPKA. A mutation in the Calpha gene that prevents myristylation allows the intracellular PKA up-regulation but blocks the ECPKA increase, suggesting that the NH(2)-terminal myristyl group of Calpha is required for the ECPKA expression. In serum of cancer patients, the ECPKA expression is up-regulated 10-fold as compared with normal serum. These results indicate that the ECPKA expression is an ordered cellular response of a living cell to actively exclude excess intracellular PKA molecules from the cell. This phenomenon is up-regulated in tumor cells and has an inverse relationship with the hormone dependency of breast cancer. Thus, the extracellular PKA may serve as a potential diagnostic and prognostic marker for cancer.
Figures



References
-
- Krebs E G, Beavo J A. Annu Rev Biochem. 1979;48:923–939. - PubMed
-
- Beebe S J, Corbin J D. The Enzymes: Control by Phosphorylation. Vol. 17. New York: Academic; 1986. pp. 43–111.
-
- McKnight G S, Clegg C H, Uhler M D, Chrivia J C, Cadd G G, Correll L A, Otten A D. Recent Prog Horm Res. 1988;44:307–335. - PubMed
-
- Levy F O, Oyen O, Sandberg M, Tasken K, Eskild W, Hansson V, Jahnsen T. Mol Endocrinol. 1988;2:1364–1373. - PubMed
-
- Lohmann S M, Walter U. In: Advances in Cyclic Nucleotide and Protein Phosphorylation Research. Greengard P, Robison G A, editors. Vol. 18. New York: Raven; 1984. pp. 63–117. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

