Aortic wall damage in mice unable to synthesize ascorbic acid
- PMID: 10639167
- PMCID: PMC15418
- DOI: 10.1073/pnas.97.2.841
Aortic wall damage in mice unable to synthesize ascorbic acid
Abstract
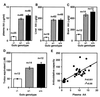
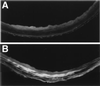
By inactivating the gene for L-gulono-gamma-lactone oxidase, a key enzyme in ascorbic acid synthesis, we have generated mice that, like humans, depend on dietary vitamin C. Regular chow, containing about 110 mg/kg of vitamin C, is unable to support the growth of the mutant mice, which require L-ascorbic acid supplemented in their drinking water (330 mg/liter). Upon withdrawal of supplementation, plasma and tissue ascorbic acid levels decreased to 10-15% of normal within 2 weeks, and after 5 weeks the mutants became anemic, began to lose weight, and die. Plasma total antioxidative capacities were approximately 37% normal in homozygotes after feeding the unsupplemented diet for 3-5 weeks. As plasma ascorbic acid decreased, small, but significant, increases in total cholesterol and decreases in high density lipoprotein cholesterol were observed. The most striking effects of the marginal dietary vitamin C were alterations in the wall of aorta, evidenced by the disruption of elastic laminae, smooth muscle cell proliferation, and focal endothelial desquamation of the luminal surface. Thus, marginal vitamin C deficiency affects the vascular integrity of mice unable to synthesize ascorbic acid, with potentially profound effects on the pathogenesis of vascular diseases. Breeding the vitamin C-dependent mice with mice carrying defined genetic mutations will provide numerous opportunities for systematic studies of the role of antioxidants in health and disease.
Figures

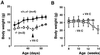


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

