Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse
- PMID: 10639174
- PMCID: PMC15425
- DOI: 10.1073/pnas.97.2.883
Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse
Abstract
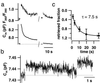
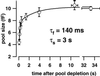
Hearing in mammals relies on the highly synchronous synaptic transfer between cochlear inner hair cells (IHCs) and the auditory nerve. We studied the presynaptic function of single mouse IHCs by monitoring membrane capacitance changes and voltage-gated Ca(2+) currents. Exocytosis initially occurred at a high rate but then slowed down within a few milliseconds, despite nearly constant Ca(2+) influx. We interpret the observed secretory depression as depletion of a readily releasable pool (RRP) of about 280 vesicles. These vesicles are probably docked close to Ca(2+) channels at the ribbon-type active zones of the IHCs. Continued depolarization evoked slower exocytosis occurring at a nearly constant rate for at least 1 s and depending on "long-distance" Ca(2+) signaling. Refilling of the RRP after depletion followed a biphasic time course and was faster than endocytosis. RRP depletion is discussed as a mechanism for fast auditory adaptation.
Figures



References
-
- Kros C J. In: The Cochlea. Dallos P, Popper A N, Fay R R, editors. New York: Springer; 1996. pp. 318–385.
-
- Kiang N Y-S, Watanabe T, Thomas E C, Clark L F. Discharge Pattern of Single Fibers in the Cat's Auditory Nerve. Cambridge, MA: MIT Press; 1965.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

