Streptococcus suis serotype 2 interactions with human brain microvascular endothelial cells
- PMID: 10639427
- PMCID: PMC97186
- DOI: 10.1128/IAI.68.2.637-643.2000
Streptococcus suis serotype 2 interactions with human brain microvascular endothelial cells
Abstract
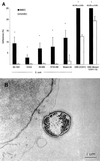
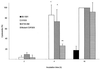
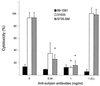
Streptococcus suis serotype 2 is a worldwide causative agent of many forms of swine infection and is also recognized as a zoonotic agent causing human disease, including meningitis. The pathogenesis of S. suis infections is poorly understood. Bacteria circulate in the bloodstream in the nonimmune host until they come in contact with brain microvascular endothelial cells (BMEC) forming the blood-brain barrier. The bacterial polysaccharide capsule confers antiphagocytic properties. It is known that group B streptococci (GBS) invade and damage BMEC, which may be a primary step in the pathogenesis of neonatal meningitis. Interactions between S. suis and human endothelial cells were studied to determine if they differ from those between GBS and endothelial cells. Invasion assays performed with BMEC and human umbilical vein endothelial cells demonstrated that unlike GBS, S. suis serotype 2 could not invade either type of cell. Adherence assays showed that S. suis adhered only to BMEC, whereas GBS adhered to both types of cell. These interactions were not affected by the presence of a capsule, since acapsular mutants from both bacterial species adhered similarly compared to the wild-type strains. Lactate dehydrogenase release measurements indicated that some S. suis strains were highly cytotoxic for BMEC, even more than GBS, whereas others were not toxic at all. Cell damage was related to suilysin (S. suis hemolysin) production, since only suilysin-producing strains were cytotoxic and cytotoxicity could be inhibited by cholesterol and antisuilysin antibodies. It is possible that hemolysin-positive S. suis strains use adherence and suilysin-induced BMEC injury, as opposed to direct cellular invasion, to proceed from the circulation to the central nervous system.
Figures





References
-
- Alouf J E, Geoffroy C. The family of the antigenically-related cholesterol-binding (“sulphydryl-activated”) cytolytic toxins. In: Alouf J E, editor. Sourcebook of bacterial protein toxins. New York, N.Y: Academic Press, Inc.; 1991. pp. 147–186.
-
- Arends J P, Zanen H C. Meningitis caused by Streptococcus suis in humans. J Infect Dis. 1988;10:131–137. - PubMed
-
- Baker C J, Edwards M S. Group B streptococcal infections. In: Klein J S, Klein J O, editors. Infectious diseases of the fetus and the newborn. Philadelphia, Pa: The W. B. Saunders Co.; 1990. pp. 742–811.
-
- Betz A L, Goldstein G W. Specialized properties and solute transport in brain capillaries. Annu Rev Physiol. 1986;48:241–250. - PubMed
-
- Boulnois G J, Paton J C, Mitchell T J, Andrew P W. Structure and function of pneumolysin, the multifunctional, thiol-activated toxin of Streptococcus pneumoniae. Mol Microbiol. 1991;5:2611–2616. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

