Identification of an antigen localized to an apparent septum within dividing chlamydiae
- PMID: 10639437
- PMCID: PMC97196
- DOI: 10.1128/IAI.68.2.708-715.2000
Identification of an antigen localized to an apparent septum within dividing chlamydiae
Abstract
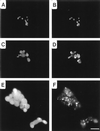
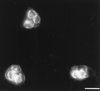
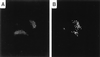
The process of chlamydial cell division has not been thoroughly investigated. The lack of detectable peptidoglycan and the absence of an FtsZ homolog within chlamydiae suggest an unusual mechanism for the division process. Our laboratory has identified an antigen (SEP antigen) localized to a ring-like structure at the apparent septum within dividing chlamydial reticulate bodies (RB). Antisera directed against SEP show similar patterns of antigen distribution in Chlamydia trachomatis and Chlamydia psittaci RB. In contrast to localization in RB, SEP in elementary bodies appears diffuse and irregular, suggesting that the distribution of the antigen is developmental-stage specific. Treatment of chlamydiae with inhibitors of peptidoglycan synthesis or culture of chlamydiae in medium lacking tryptophan leads to the formation of nondividing, aberrant RB. Staining of aberrant RB with anti-SEP reveals a marked redistribution of the antigen. Within C. trachomatis-infected cells, ampicillin treatment leads to high levels of SEP accumulation at the periphery of aberrant RB, while in C. psittaci, treatment causes SEP to localize to distinct punctate sites within the bacteria. Aberrancy produced via tryptophan depletion results in a different pattern of SEP distribution. In either case, the reversal of aberrant formation results in the production of normal RB and a redistribution of SEP to the apparent plane of bacterial division. Collectively these studies identify a unique chlamydial-genus-common and developmental-stage-specific antigen that may be associated with RB division.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

