Immunogenicity of the B monomer of Escherichia coli heat-labile toxin expressed on the surface of Streptococcus gordonii
- PMID: 10639444
- PMCID: PMC97203
- DOI: 10.1128/IAI.68.2.760-766.2000
Immunogenicity of the B monomer of Escherichia coli heat-labile toxin expressed on the surface of Streptococcus gordonii
Abstract
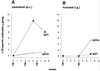
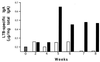
The B monomer of the Escherichia coli heat-labile toxin (LTB) was expressed on the surface of the human oral commensal bacterium Streptococcus gordonii. Recombinant bacteria expressing LTB were used to immunize BALB/c mice subcutaneously and intragastrically. The LTB monomer expressed on the streptococcal surface proved to be highly immunogenic, as LTB-specific immunoglobulin G (IgG) serum titers of 140,000 were induced after systemic immunization. Most significantly, these antibodies were capable of neutralizing the enterotoxin in a cell neutralization assay. Following mucosal delivery, antigen-specific IgA antibodies were found in feces and antigen-specific IgG antibodies were found in sera. Analysis of serum IgG subclasses showed a clear predominance of IgG1 when recombinant bacteria were inoculated subcutaneously, while a prevalence of IgG2a was observed upon intragastric delivery, suggesting, in this case, the recruitment of a Th1 type of immune response.
Figures

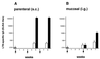
References
-
- Amin T, Hirst T R. Purification of the B-subunit oligomer of Escherichia coli heat-labile enterotoxin by heterologous expression and secretion in a marine vibrio. Protein Expr Purif. 1994;5:198–204. - PubMed
-
- Apter F M, Michetti P, Winner III L S, Mack J A, Mekalanos J J, Neutra M R. Analysis of the role of antilipopolysaccharide and anti-cholera toxin immunoglobulin A antibodies in protection against Vibrio cholerae and cholera toxin by use of monoclonal IgA antibodies in vivo. Infect Immun. 1993;61:5279–5285. - PMC - PubMed
-
- Backstrom M, Shahabi V, Johansson S, Teneberg S, Kjellberg A, Miller-Podraza H, Holmgren J, Lebens M. Structural basis for differential receptor binding of cholera and Escherichia coli heat-labile toxins: influence of heterologous amino acid substitutions in the cholera B-subunit. Mol Microbiol. 1997;24:489–497. - PubMed
-
- Chamberlain L, Wells J M, Robinson K, Schofield K, Le Page R. Mucosal immunization with recombinant Lactococcus lactis. In: Pozzi G, Wells J M, editors. Gram-positive bacteria. Vaccine vehicles for mucosal immunization. Austin, Tex: RG Landes; 1997. pp. 83–106.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

