Disruption of the genes encoding antigen 85A and antigen 85B of Mycobacterium tuberculosis H37Rv: effect on growth in culture and in macrophages
- PMID: 10639445
- PMCID: PMC97204
- DOI: 10.1128/IAI.68.2.767-778.2000
Disruption of the genes encoding antigen 85A and antigen 85B of Mycobacterium tuberculosis H37Rv: effect on growth in culture and in macrophages
Abstract
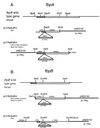
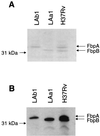
The mechanism of pathogenesis of Mycobacterium tuberculosis is thought to be multifactorial. Among the putative virulence factors is the antigen 85 (Ag85) complex. This family of exported fibronectin-binding proteins consists of members Ag85A, Ag85B, and Ag85C and is most prominently represented by 85A and 85B. These proteins have recently been shown to possess mycolyl transferase activity and likely play a role in cell wall synthesis. The purpose of this study was to generate strains of M. tuberculosis deficient in expression of the principal members of this complex in order to determine their role in the pathogenesis of M. tuberculosis. Constructs of fbpA and fbpB disrupted with the kanamycin resistance marker OmegaKm and containing varying amounts of flanking gene and plasmid vector sequences were then introduced as linear fragments into H37Rv by electroporation. Southern blot and PCR analyses revealed disruption of the homologous gene locus in one fbpA::OmegaKm transformant and one fbpB::OmegaKm transformant. The fbpA::OmegaKm mutant, LAa1, resulted from a double-crossover integration event, whereas the fbpB::OmegaKm variant, LAb1, was the product of a single-crossover type event that resulted in insertion of both OmegaKm and plasmid sequences. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis confirmed that expression of the disrupted gene was not detectable in the fbpA and fbpB mutants. Analysis of growth rates demonstrated that the fbpB mutant LAb1 grew at a rate similar to that of the wild-type parent in enriched and nutrient-poor laboratory media as well as in human (THP-1) and mouse (J774.1A) macrophage-like cell lines. The fbpA mutant LAa1 grew similarly to the parent H37Rv in enriched laboratory media but exhibited little or no growth in nutrient-poor media and macrophage-like cell lines. The targeted disruption of two genes encoding mycolyl transferase and fibronectin-binding activities in M. tuberculosis will permit the systematic determination of their roles in the physiology and pathogenesis of this organism.
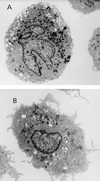
Figures




References
-
- Azad A K, Sirakova T D, Fernandes N D, Kolattukudy P E. Gene knockout reveals a novel gene cluster for the synthesis of a class of cell wall lipids unique to pathogenic mycobacteria. J Biol Chem. 1997;272:16741–16745. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

