The porcine humoral immune response against pseudorabies virus specifically targets attachment sites on glycoprotein gC
- PMID: 10644346
- PMCID: PMC111651
- DOI: 10.1128/jvi.74.4.1752-1760.2000
The porcine humoral immune response against pseudorabies virus specifically targets attachment sites on glycoprotein gC
Abstract
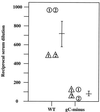
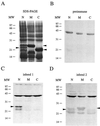
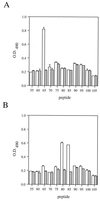
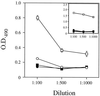
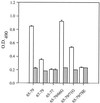
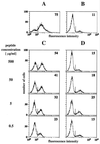
High titers of virus-neutralizing antibodies directed against glycoprotein gC of Pseudorabies virus (PRV) (Suid herpesvirus 1) are generally observed in the serum of immunized pigs. A known function of the glycoprotein gC is to mediate attachment of PRV to target cells through distinct viral heparin-binding domains (HBDs). Therefore, it was suggested that the virus-neutralizing activity of anti-PRV sera is directed against HBDs on gC. To address this issue, sera with high virus-neutralizing activity against gC were used to characterize the anti-gC response. Epitope mapping demonstrated that amino acids of HBDs are part of an antigenic antibody binding domain which is located in the N-terminal part of gC. Binding of antibodies to this antigenic domain of gC was further shown to interfere with the viral attachment. Therefore, these results show that the viral HBDs are accessible targets for the humoral anti-PRV response even after tolerance induction against self-proteins, which utilize similar HBDs to promote host protein-protein interactions. The findings indicate that the host's immune system can specifically block the attachment function of PRV gC. Since HBDs promote the attachment of a number of herpesviruses, the design of future antiherpesvirus vaccines should aim to induce a humoral immune response that prevents HBD-mediated viral attachment.
Figures

Similar articles
-
The receptor-binding domain of pseudorabies virus glycoprotein gC is composed of multiple discrete units that are functionally redundant.J Virol. 1996 Mar;70(3):1355-64. doi: 10.1128/JVI.70.3.1355-1364.1996. J Virol. 1996. PMID: 8627651 Free PMC article.
-
Pseudorabies virus glycoprotein gI: in vitro and in vivo analysis of immunorelevant epitopes.J Gen Virol. 1990 May;71 ( Pt 5):1141-51. doi: 10.1099/0022-1317-71-5-1141. J Gen Virol. 1990. PMID: 1693164
-
Additional Insertion of gC Gene Triggers Better Immune Efficacy of TK/gI/gE-Deleted Pseudorabies Virus in Mice.Viruses. 2024 Apr 29;16(5):706. doi: 10.3390/v16050706. Viruses. 2024. PMID: 38793591 Free PMC article.
-
Live attenuated pseudorabies virus expressing envelope glycoprotein E1 of hog cholera virus protects swine against both pseudorabies and hog cholera.J Virol. 1991 May;65(5):2761-5. doi: 10.1128/JVI.65.5.2761-2765.1991. J Virol. 1991. PMID: 1850051 Free PMC article.
-
Protective effect of glycoprotein gC-rich antigen against pseudorabies virus.J Vet Med Sci. 1997 Aug;59(8):657-63. doi: 10.1292/jvms.59.657. J Vet Med Sci. 1997. PMID: 9300361
Cited by
-
Glycoproteins C and D of PRV Strain HB1201 Contribute Individually to the Escape From Bartha-K61 Vaccine-Induced Immunity.Front Microbiol. 2020 Mar 10;11:323. doi: 10.3389/fmicb.2020.00323. eCollection 2020. Front Microbiol. 2020. PMID: 32210933 Free PMC article.
-
Genetic characterization and mutation analysis of Qihe547 Aujeszky's disease virus in China.BMC Vet Res. 2018 Jul 6;14(1):218. doi: 10.1186/s12917-018-1492-2. BMC Vet Res. 2018. PMID: 29980205 Free PMC article.
-
Molecular epidemiology of outbreak-associated pseudorabies virus (PRV) strains in central China.Virus Genes. 2015 Jun;50(3):401-9. doi: 10.1007/s11262-015-1190-0. Epub 2015 Apr 10. Virus Genes. 2015. PMID: 25860998
-
Novel recombinant parapoxvirus vectors induce protective humoral and cellular immunity against lethal herpesvirus challenge infection in mice.J Virol. 2003 Sep;77(17):9312-23. doi: 10.1128/jvi.77.17.9312-9323.2003. J Virol. 2003. PMID: 12915547 Free PMC article.
-
Anti-glycoprotein H antibody impairs the pathogenicity of varicella-zoster virus in skin xenografts in the SCID mouse model.J Virol. 2010 Jan;84(1):141-52. doi: 10.1128/JVI.01338-09. J Virol. 2010. PMID: 19828615 Free PMC article.
References
-
- Ben-Porat T, DeMarchi J M, Lomniczi B, Kaplan A S. Role of glycoproteins of pseudorabies virus in eliciting neutralizing antibodies. Virology. 1986;154:325–334. - PubMed
-
- Cardin A D, Weintraub H J R. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989;9:21–32. - PubMed
-
- Compton T, Nowlin D, Cooper N R. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan-sulfate. Virology. 1993;193:834–841. - PubMed
-
- Cornall R J, Goodnow C C, Cyster J G. The regulation of self-reactive B cells. Curr Opin Immunol. 1995;7:804–811. - PubMed
-
- Davis-Poynter N J, Farrell H E. Masters of deception: a review of herpesvirus immune evasion strategies. Immunol Cell Biol. 1996;74:513–522. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

