Oligomeric modeling and electrostatic analysis of the gp120 envelope glycoprotein of human immunodeficiency virus
- PMID: 10644369
- PMCID: PMC111674
- DOI: 10.1128/jvi.74.4.1961-1972.2000
Oligomeric modeling and electrostatic analysis of the gp120 envelope glycoprotein of human immunodeficiency virus
Abstract
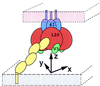
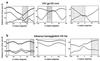
The human immunodeficiency virus envelope glycoproteins, gp120 and gp41, function in cell entry by binding to CD4 and a chemokine receptor on the cell surface and orchestrating the direct fusion of the viral and target cell membranes. On the virion surface, three gp120 molecules associate noncovalently with the ectodomain of the gp41 trimer to form the envelope oligomer. Although an atomic-level structure of a monomeric gp120 core has been determined, the structure of the oligomer is unknown. Here, the orientation of gp120 in the oligomer is modeled by using quantifiable criteria of carbohydrate exposure, occlusion of conserved residues, and steric considerations with regard to the binding of the neutralizing antibody 17b. Applying similar modeling techniques to influenza virus hemagglutinin suggests a rotational accuracy for the oriented gp120 of better than 10 degrees. The model shows that CD4 binds obliquely, such that multiple CD4 molecules bound to the same oligomer have their membrane-spanning portions separated by at least 190 A. The chemokine receptor, in contrast, binds to a sterically restricted surface close to the trimer axis. Electrostatic analyses reveal a basic region which faces away from the virus, toward the target cell membrane, and is conserved on core gp120. The electrostatic potentials of this region are strongly influenced by the overall charge, but not the precise structure, of the third variable (V3) loop. This dependence on charge and not structure may make electrostatic interactions between this basic region and the cell difficult to target therapeutically and may also provide a means of viral escape from immune system surveillance.
Figures




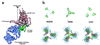
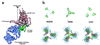
Similar articles
-
A V3 loop-dependent gp120 element disrupted by CD4 binding stabilizes the human immunodeficiency virus envelope glycoprotein trimer.J Virol. 2010 Apr;84(7):3147-61. doi: 10.1128/JVI.02587-09. Epub 2010 Jan 20. J Virol. 2010. PMID: 20089638 Free PMC article.
-
The antigenic structure of the HIV gp120 envelope glycoprotein.Nature. 1998 Jun 18;393(6686):705-11. doi: 10.1038/31514. Nature. 1998. PMID: 9641684
-
Activation and Inactivation of Primary Human Immunodeficiency Virus Envelope Glycoprotein Trimers by CD4-Mimetic Compounds.J Virol. 2017 Jan 18;91(3):e01880-16. doi: 10.1128/JVI.01880-16. Print 2017 Feb 1. J Virol. 2017. PMID: 27881646 Free PMC article.
-
Functional analysis of the disulfide-bonded loop/chain reversal region of human immunodeficiency virus type 1 gp41 reveals a critical role in gp120-gp41 association.J Virol. 2001 Jul;75(14):6635-44. doi: 10.1128/JVI.75.14.6635-6644.2001. J Virol. 2001. PMID: 11413331 Free PMC article.
-
CD4 activation of HIV fusion.Int J Cell Cloning. 1992 Nov;10(6):323-32. doi: 10.1002/stem.5530100603. Int J Cell Cloning. 1992. PMID: 1281202 Review.
Cited by
-
Dynamic domains and geometrical properties of HIV-1 gp120 during conformational changes induced by CD4 binding.J Mol Model. 2007 Mar;13(3):411-24. doi: 10.1007/s00894-006-0158-3. Epub 2006 Nov 28. J Mol Model. 2007. PMID: 17131136
-
Selective interactions of polyanions with basic surfaces on human immunodeficiency virus type 1 gp120.J Virol. 2000 Feb;74(4):1948-60. doi: 10.1128/jvi.74.4.1948-1960.2000. J Virol. 2000. PMID: 10644368 Free PMC article.
-
Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1.J Virol. 2003 Oct;77(19):10557-65. doi: 10.1128/jvi.77.19.10557-10565.2003. J Virol. 2003. PMID: 12970440 Free PMC article.
-
The human immunodeficiency virus type 1 envelope spike of primary viruses can suppress antibody access to variable regions.J Virol. 2009 Feb;83(4):1649-59. doi: 10.1128/JVI.02046-08. Epub 2008 Nov 26. J Virol. 2009. PMID: 19036813 Free PMC article.
-
CCR5 inhibitors: Emerging promising HIV therapeutic strategy.Indian J Sex Transm Dis AIDS. 2009 Jan;30(1):1-9. doi: 10.4103/0253-7184.55471. Indian J Sex Transm Dis AIDS. 2009. PMID: 21938106 Free PMC article.
References
-
- Altschul S, Gish W, Miller W, Myer E, Lipman D. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Baba M, Nakajima M, Schols D, Pauwels R, Balzarini J, De Clercq E. Pentosan polysulfate, a sulfated oligosaccharide, is a potent and selective anti-HIV agent in vitro. Antivir Res. 1988;9:335–343. - PubMed
-
- Bizebard T, Benoit G, Rigolet P, Rasmussen B, Diat O, Bosecke P, Wharton S, Skehel J, Knossow M. Structure of influenza virus haemagglutinin complexed with a neutralizing antibody. Nature. 1995;376:92–94. - PubMed
-
- Brunger A T. X-PLOR version 3.1 manual. New Haven, Conn: Yale University; 1993.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

