Uterine dysfunction and genetic modifiers in centromere protein B-deficient mice
- PMID: 10645947
- PMCID: PMC310504
Uterine dysfunction and genetic modifiers in centromere protein B-deficient mice
Abstract
Centromere protein B (CENP-B) binds constitutively to mammalian centromere repeat DNA and is highly conserved between humans and mouse. Cenpb null mice appear normal but have lower body and testis weights. We demonstrate here that testis-weight reduction is seen in male null mice generated on three different genetic backgrounds (denoted R1, W9.5, and C57), whereas body-weight reduction is dependent on the genetic background as well as the gender of the animals. In addition, Cenpb null females show 31%, 33%, and 44% reduced uterine weights on the R1, W9.5, and C57 backgrounds, respectively. Production of "revertant" mice lacking the targeted frameshift mutation but not the other components of the targeting construct corrected these differences, indicating that the observed phenotype is attributable to Cenpb gene disruption rather than a neighbouring gene effect induced by the targeting construct. The R1 and W9.5 Cenpb null females are reproductively competent but show age-dependent reproductive deterioration leading to a complete breakdown at or before 9 months of age. Reproductive dysfunction is much more severe in the C57 background as Cenpb null females are totally incompetent or are capable of producing no more than one litter. These results implicate a further genetic modifier effect on female reproductive performance. Histology of the uterus reveals normal myometrium and endometrium but grossly disrupted luminal and glandular epithelium. Tissue in situ hybridization demonstrates high Cenpb expression in the uterine epithelium of wild-type animals. This study details the first significant phenotype of Cenpb gene disruption and suggests an important role of Cenpb in uterine morphogenesis and function that may have direct implications for human reproductive pathology.
Figures




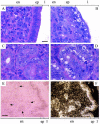
Similar articles
-
Centromere protein b-null mice display decreasing reproductive performance through successive generations of breeding due to diminishing endometrial glands.Reproduction. 2004 Mar;127(3):367-77. doi: 10.1530/rep.1.00102. Reproduction. 2004. PMID: 15016956
-
Centromere protein B null mice are mitotically and meiotically normal but have lower body and testis weights.J Cell Biol. 1998 Apr 20;141(2):309-19. doi: 10.1083/jcb.141.2.309. J Cell Biol. 1998. PMID: 9548711 Free PMC article.
-
The cenpB gene is not essential in mice.Chromosoma. 1998 Dec;107(8):570-6. doi: 10.1007/s004120050343. Chromosoma. 1998. PMID: 9933410
-
Estrogen receptor gene disruption: molecular characterization and experimental and clinical phenotypes.Recent Prog Horm Res. 1996;51:159-86; discussion 186-8. Recent Prog Horm Res. 1996. PMID: 8701078 Review.
-
Mammalian artificial chromosomes and chromosome transgenics.Trends Genet. 1997 Sep;13(9):345-7. doi: 10.1016/s0168-9525(97)01256-0. Trends Genet. 1997. PMID: 9287486 Review. No abstract available.
Cited by
-
Crystal structure of the CENP-B protein-DNA complex: the DNA-binding domains of CENP-B induce kinks in the CENP-B box DNA.EMBO J. 2001 Dec 3;20(23):6612-8. doi: 10.1093/emboj/20.23.6612. EMBO J. 2001. PMID: 11726497 Free PMC article.
-
Functional redundancies, distinct localizations and interactions among three fission yeast homologs of centromere protein-B.Genetics. 2001 Mar;157(3):1191-203. doi: 10.1093/genetics/157.3.1191. Genetics. 2001. PMID: 11238404 Free PMC article.
-
DNA Sequence-Specific Binding of CENP-B Enhances the Fidelity of Human Centromere Function.Dev Cell. 2015 May 4;33(3):314-27. doi: 10.1016/j.devcel.2015.03.020. Dev Cell. 2015. PMID: 25942623 Free PMC article.
-
Centromeres of filamentous fungi.Chromosome Res. 2012 Jul;20(5):635-56. doi: 10.1007/s10577-012-9290-3. Chromosome Res. 2012. PMID: 22752455 Free PMC article. Review.
-
Partially functional Cenpa-GFP fusion protein causes increased chromosome missegregation and apoptosis during mouse embryogenesis.Chromosome Res. 2003;11(4):345-57. doi: 10.1023/a:1024044008009. Chromosome Res. 2003. PMID: 12906131
References
-
- Banbury Conference Consortium. Neuron. 1997;19:755–759. - PubMed
-
- Bejarano LA, Valdivia MM. Molecular cloning of an intronless gene for the hamster centromere antigen CENP-B. Biochim Biophys Acta. 1996;1307:21–25. - PubMed
-
- Bronson FH, Dagg CP, Snell GD. Reproduction. In: Green EL, editor. Biology of the laboratory mouse. New York, NY: McGraw-Hill; 1966. pp. 187–204.
-
- Buzin CH, Mann JR, Singer-Sam J. Quantitative RT-PCR assays show Xist RNA levels are low in female adult mouse tissue, embryos and embryoid bodies. Development. 1994;120:3529–3536. - PubMed
-
- Choo KHA. The centromere. Oxford, UK: Oxford University Press; 1997a.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
