Mu-opioid agonist inhibition of kappa-opioid receptor-stimulated extracellular signal-regulated kinase phosphorylation is dynamin-dependent in C6 glioma cells
- PMID: 10646508
- PMCID: PMC2571950
- DOI: 10.1046/j.1471-4159.2000.740574.x
Mu-opioid agonist inhibition of kappa-opioid receptor-stimulated extracellular signal-regulated kinase phosphorylation is dynamin-dependent in C6 glioma cells
Abstract
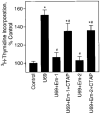
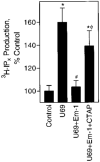
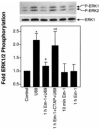
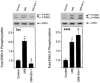
In previous studies we found that mu-opioids, acting via mu-opioid receptors, inhibit endothelin-stimulated C6 glioma cell growth. In the preceding article we show that the kappa-selective opioid agonist U69,593 acts as a mitogen with a potency similar to that of endothelin in the same astrocytic model system. Here we report that C6 cell treatment with mu-opioid agonists for 1 h results in the inhibition of kappa-opioid mitogenic signaling. The mu-selective agonist endomorphin-1 attenuates kappa-opioid-stimulated DNA synthesis, phosphoinositide turnover, and extracellular signal-regulated kinase phosphorylation. To investigate the role of receptor endocytosis in signaling, we have examined the effects of dynamin-1 and its GTPase-defective, dominant suppressor mutant (K44A) on opioid modulation of extracellular signal-regulated kinase phosphorylation in C6 cells. Overexpression of dynamin K44A in C6 cells does not affect kappa-opioid phosphorylation of extracellular signal-regulated kinase. However, it does block the inhibitory action on kappa-opioid signaling mediated by the kappa-opioid receptor. Our results are consistent with a growing body of evidence of the opposing actions of mu- and kappa-opioids and provide new insight into the role of opioid receptor trafficking in signaling.
Figures

References
-
- Ahn S, Maudsley S, Luttrel LM, Lefkowitz RJ, Daaka Y. Src-mediated tyrosine phosphorylation of dynamin is required for β2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J. Biol. Chem. 1999;274:1185–1188. - PubMed
-
- Albouz S, Tocque B, Hauw JJ, Boutry JM, LeSaux F, Bourdoin R, Baumann N. Tricyclic antidepressant desipramine induces stereospecific opiate binding and lipid modification in rat glioma C6 cells. Life Sci. 1982;31:2549–2554. - PubMed
-
- Barg J, Belcheva M, Bem WT, Lambourne B, McLachlan JA, Tolman KC, Johnson FE, Coscia CJ. Desipramine modulation of sigma and opioid peptide receptor expression in glial cells. Peptides. 1991;12:845–849. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

