Production of an endo-beta-N-acetylglucosaminidase activity mediates growth of Enterococcus faecalis on a high-mannose-type glycoprotein
- PMID: 10648510
- PMCID: PMC94360
- DOI: 10.1128/JB.182.4.882-890.2000
Production of an endo-beta-N-acetylglucosaminidase activity mediates growth of Enterococcus faecalis on a high-mannose-type glycoprotein
Abstract
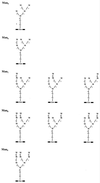
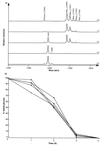
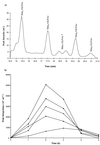
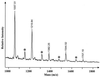
Enterococcus faecalis is associated with a high proportion of nosocomial infections; however, little is known of the ability of this organism to proliferate in vivo. The ability of RNase B, a model glycoprotein with a single N-glycosylation site occupied by a family of high-mannose-type glycans (Man(5)- to Man(9)-GlcNAc(2)), to support growth of E. faecalis was investigated. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of RNase B demonstrated a reduction in the molecular mass of this glycoprotein during bacterial growth. Further analysis by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry revealed that this mass shift was due to the degradation of all high-mannose-type glycoforms to a single N-linked N-acetylglucosamine residue. High-pH anion-exchange chromatography analysis during exponential growth demonstrated the presence of RNase B-derived glycans in the culture supernatant, indicating the presence of an endoglycosidase activity. The free glycans were eluted with the same retention times as those generated by the action of Streptomyces plicatus endo-beta-N-acetylglucosaminidase H on RNase B. The cleavage specificity was confirmed by MALDI-TOF analysis of the free glycans, which showed glycan species containing only one N-acetylglucosamine residue. No free glycans were detectable after 5 h of bacterial growth, and we have subsequently demonstrated the presence of mannosidase activity in E. faecalis, which releases free mannose from RNase B-derived glycans. We propose that this deglycosylation of glycoproteins containing high-mannose-type glycans and the subsequent degradation of the released glycans by E. faecalis may play a role in the survival and persistence of this nosocomial pathogen in vivo.
Figures

Similar articles
-
An endo-β-N-acetylglucosaminidase from Enterococcus faecalis V583 responsible for the hydrolysis of high-mannose and hybrid-type N-linked glycans.FEMS Microbiol Lett. 2011 Dec;325(2):123-9. doi: 10.1111/j.1574-6968.2011.02419.x. Epub 2011 Oct 4. FEMS Microbiol Lett. 2011. PMID: 22093069
-
Distribution of endo-beta-N-acetylglucosaminidase amongst enterococci.J Med Microbiol. 2001 Jul;50(7):620-626. doi: 10.1099/0022-1317-50-7-620. J Med Microbiol. 2001. PMID: 11444772
-
Detecting mannosidase activities using ribonuclease B and matrix-assisted laser desorption/ionization-time of flight mass spectrometry.Anal Biochem. 2000 Jul 1;282(2):165-72. doi: 10.1006/abio.2000.4606. Anal Biochem. 2000. PMID: 10873270
-
Mammalian alpha-mannosidases--multiple forms but a common purpose?Glycobiology. 1994 Oct;4(5):551-66. doi: 10.1093/glycob/4.5.551. Glycobiology. 1994. PMID: 7881169 Review.
-
Structural features of free N-glycans occurring in plants and functional features of de-N-glycosylation enzymes, ENGase, and PNGase: the presence of unusual plant complex type N-glycans.Front Plant Sci. 2014 Sep 4;5:429. doi: 10.3389/fpls.2014.00429. eCollection 2014. Front Plant Sci. 2014. PMID: 25237315 Free PMC article. Review.
Cited by
-
New roles in hemicellulosic sugar fermentation for the uncultivated Bacteroidetes family BS11.ISME J. 2017 Mar;11(3):691-703. doi: 10.1038/ismej.2016.150. Epub 2016 Dec 13. ISME J. 2017. PMID: 27959345 Free PMC article.
-
Endo-β-N-acetylglucosaminidases from infant gut-associated bifidobacteria release complex N-glycans from human milk glycoproteins.Mol Cell Proteomics. 2012 Sep;11(9):775-85. doi: 10.1074/mcp.M112.018119. Epub 2012 Jun 27. Mol Cell Proteomics. 2012. PMID: 22745059 Free PMC article.
-
Mannosidase production by viridans group streptococci.J Clin Microbiol. 2001 Mar;39(3):995-1001. doi: 10.1128/JCM.39.3.995-1001.2001. J Clin Microbiol. 2001. PMID: 11230417 Free PMC article.
-
Host Cell Glycocalyx Remodeling Reveals SARS-CoV-2 Spike Protein Glycomic Binding Sites.Front Mol Biosci. 2022 Mar 14;9:799703. doi: 10.3389/fmolb.2022.799703. eCollection 2022. Front Mol Biosci. 2022. PMID: 35372520 Free PMC article.
-
Structural bases for N-glycan processing by mannoside phosphorylase.Acta Crystallogr D Biol Crystallogr. 2015 Jun;71(Pt 6):1335-46. doi: 10.1107/S1399004715006604. Epub 2015 May 14. Acta Crystallogr D Biol Crystallogr. 2015. PMID: 26057673 Free PMC article.
References
-
- Beighton D, Hardie J M, Whiley R A. A scheme for the identification of viridans streptococci. J Med Microbiol. 1991;35:367–372. - PubMed
-
- Byers H L, Homer K A, Beighton D. Utilization of sialic acid by viridans streptococci. J Dent Res. 1996;75:1564–1571. - PubMed
-
- Byers H L, Tarelli E, Homer K A, Beighton D. Sequential deglycosylation and utilization of the N-linked, complex-type glycans of human α1-acid glycoprotein mediates growth of Streptococcus oralis. Glycobiology. 1999;9:469–479. - PubMed
-
- Chenoweth C, Schaberg D. The epidemiology of enterococci. Eur J Clin Microbiol Infect Dis. 1990;9:80–89. - PubMed
-
- Corfield A P, Wagner S A, Clamp J R, Kriaris M S, Hoskins L C. Mucin degradation in the human colon: production of sialidase, sialate O-acetylesterase, N-acetylneuraminate lyase, arylesterase, and glycosulfatase activities by strains of fecal bacteria. Infect Immun. 1992;60:3971–3978. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

