Nuclear eukaryotic initiation factor 4E (eIF4E) colocalizes with splicing factors in speckles
- PMID: 10648556
- PMCID: PMC2174286
- DOI: 10.1083/jcb.148.2.239
Nuclear eukaryotic initiation factor 4E (eIF4E) colocalizes with splicing factors in speckles
Abstract
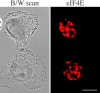
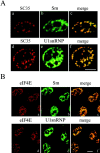
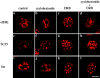
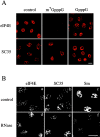
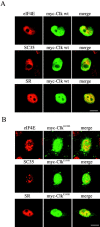
The eukaryotic initiation factor 4E (eIF4E) plays a pivotal role in the control of protein synthesis. eIF4E binds to the mRNA 5' cap structure, m(7)GpppN (where N is any nucleotide) and promotes ribosome binding to the mRNA. It was previously shown that a fraction of eIF4E localizes to the nucleus (Lejbkowicz, F., C. Goyer, A. Darveau, S. Neron, R. Lemieux, and N. Sonenberg. 1992. Proc. Natl. Acad. Sci. USA. 89:9612-9616). Here, we show that the nuclear eIF4E is present throughout the nucleoplasm, but is concentrated in speckled regions. Double label immunofluorescence confocal microscopy shows that eIF4E colocalizes with Sm and U1snRNP. We also demonstrate that eIF4E is specifically released from the speckles by the cap analogue m(7)GpppG in a cell permeabilization assay. However, eIF4E is not released from the speckles by RNase A treatment, suggesting that retention of eIF4E in the speckles is not RNA-mediated. 5,6-dichloro-1-beta-d-ribofuranosylbenzimidazole (DRB) treatment of cells causes the condensation of eIF4E nuclear speckles. In addition, overexpression of the dual specificity kinase, Clk/Sty, but not of the catalytically inactive form, results in the dispersion of eIF4E nuclear speckles.
Figures
References
-
- Beyer A.L., Osheim Y.N. Splice site selection, rate of splicing, and alternative splicing on nascent transcripts. Genes Dev. 1988;2:754–765 . - PubMed
-
- Bringmann P., Rinke J., Appel B., Reuter R., Luhrmann R. Purification of snRNPs U1, U2, U4, U5 and U6 with 2,2,7-trimethylguanosine-specific antibody and definition of their constituent proteins reacting with anti-Sm and anti-(U1)RNP antisera. EMBO (Eur. Mol. Biol. Organ.) J. 1983;2:1129–1135 . - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

