An ATP-dependent, Ran-independent mechanism for nuclear import of the U1A and U2B" spliceosome proteins
- PMID: 10648562
- PMCID: PMC2174293
- DOI: 10.1083/jcb.148.2.293
An ATP-dependent, Ran-independent mechanism for nuclear import of the U1A and U2B" spliceosome proteins
Abstract
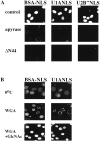
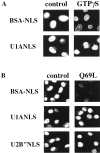
Nuclear import of the two uracil-rich small nuclear ribonucleoprotein (U snRNP) components U1A and U2B" is mediated by unusually long and complex nuclear localization signals (NLSs). Here we investigate nuclear import of U1A and U2B" in vitro and demonstrate that it occurs by an active, saturable process. Several lines of evidence suggest that import of the two proteins occurs by an import mechanism different to those characterized previously. No cross competition is seen with a variety of previously studied NLSs. In contrast to import mediated by members of the importin-beta family of nucleocytoplasmic transport receptors, U1A/U2B" import is not inhibited by either nonhydrolyzable guanosine triphosphate (GTP) analogues or by a mutant of the GTPase Ran that is incapable of GTP hydrolysis. Adenosine triphosphate is capable of supporting U1A and U2B" import, whereas neither nonhydrolyzable adenosine triphosphate analogues nor GTP can do so. U1A and U2B" import in vitro does not require the addition of soluble cytosolic proteins, but a factor or factors required for U1A and U2B" import remains tightly associated with the nuclear fraction of conventionally permeabilized cells. This activity can be solubilized in the presence of elevated MgCl(2). These data suggest that U1A and U2B" import into the nucleus occurs by a hitherto uncharacterized mechanism.
Figures






Similar articles
-
Target discrimination by RNA-binding proteins: role of the ancillary protein U2A' and a critical leucine residue in differentiating the RNA-binding specificity of spliceosomal proteins U1A and U2B".RNA. 1998 Nov;4(11):1386-96. doi: 10.1017/s1355838298981171. RNA. 1998. PMID: 9814759 Free PMC article.
-
In vitro and in vivo evidence that protein and U1 snRNP nuclear import in somatic cells differ in their requirement for GTP-hydrolysis, Ran/TC4 and RCC1.Nucleic Acids Res. 1996 May 15;24(10):1829-36. doi: 10.1093/nar/24.10.1829. Nucleic Acids Res. 1996. PMID: 8657562 Free PMC article.
-
The importin-beta binding domain of snurportin1 is responsible for the Ran- and energy-independent nuclear import of spliceosomal U snRNPs in vitro.J Cell Biol. 2002 Feb 4;156(3):467-79. doi: 10.1083/jcb.200108114. Epub 2002 Jan 28. J Cell Biol. 2002. PMID: 11815630 Free PMC article.
-
How proteins enter the nucleus.Cell. 1991 Feb 8;64(3):489-97. doi: 10.1016/0092-8674(91)90233-o. Cell. 1991. PMID: 1991319 Review.
-
Nuclear import and export: transport factors, mechanisms and regulation.Crit Rev Eukaryot Gene Expr. 1999;9(2):89-106. doi: 10.1615/critreveukargeneexpr.v9.i2.10. Crit Rev Eukaryot Gene Expr. 1999. PMID: 10445152 Review.
Cited by
-
ALADINI482S causes selective failure of nuclear protein import and hypersensitivity to oxidative stress in triple A syndrome.Proc Natl Acad Sci U S A. 2006 Feb 14;103(7):2298-303. doi: 10.1073/pnas.0505598103. Epub 2006 Feb 7. Proc Natl Acad Sci U S A. 2006. PMID: 16467144 Free PMC article.
-
Comparative Proteomic Analysis of Drug Trichosanthin Addition to BeWo Cell Line.Molecules. 2022 Feb 28;27(5):1603. doi: 10.3390/molecules27051603. Molecules. 2022. PMID: 35268705 Free PMC article.
-
Avian reovirus sigmaA localizes to the nucleolus and enters the nucleus by a nonclassical energy- and carrier-independent pathway.J Virol. 2009 Oct;83(19):10163-75. doi: 10.1128/JVI.01080-09. Epub 2009 Jul 29. J Virol. 2009. PMID: 19640987 Free PMC article.
-
An in vitro reconstituted U1 snRNP allows the study of the disordered regions of the particle and the interactions with proteins and ligands.Nucleic Acids Res. 2021 Jun 21;49(11):e63. doi: 10.1093/nar/gkab135. Nucleic Acids Res. 2021. PMID: 33677607 Free PMC article.
-
Biallelic variants in SNUPN cause a limb girdle muscular dystrophy with myofibrillar-like features.Brain. 2024 Aug 1;147(8):2867-2883. doi: 10.1093/brain/awae046. Brain. 2024. PMID: 38366623 Free PMC article.
References
-
- Adam S.A., Gerace L. Cytosolic proteins that specifically bind nuclear location signals are receptors for nuclear import. Cell. 1991;66:837–847 . - PubMed
-
- Adam S.A., Sterne-Marr R., Gerace L. In vitro nuclear protein import using permeabilized mammalian cells. Methods Cell Biol. 1991;35:469–482 . - PubMed
-
- Arts G.J., Fornerod M., Mattaj I.W. Identification of a nuclear export receptor for tRNA. Curr. Biol. 1998;8:305–314 . - PubMed
-
- Bonner W.M. Protein migration and accumulation in nuclei. In: Busch H., editor. The Cell Nucleus. Vol. 6. Academic Press; New York : 1978. pp. 97–148.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

