Caenorhabditis elegans UNC-45 is a component of muscle thick filaments and colocalizes with myosin heavy chain B, but not myosin heavy chain A
- PMID: 10648570
- PMCID: PMC2174295
- DOI: 10.1083/jcb.148.2.375
Caenorhabditis elegans UNC-45 is a component of muscle thick filaments and colocalizes with myosin heavy chain B, but not myosin heavy chain A
Abstract
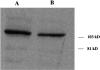
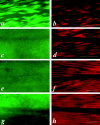
In the nematode Caenorhabditis elegans, animals mutant in the gene encoding the protein product of the unc-45 gene (UNC-45) have disorganized muscle thick filaments in body wall muscles. Although UNC-45 contains tetratricopeptide repeats (TPR) as well as limited similarity to fungal proteins, no biochemical role has yet been found. UNC-45 reporters are expressed exclusively in muscle cells, and a functional reporter fusion is localized in the body wall muscles in a pattern identical to thick filament A-bands. UNC-45 colocalizes with myosin heavy chain (MHC) B in wild-type worms as well as in temperature-sensitive (ts) unc-45 mutants, but not in a mutant in which MHC B is absent. Surprisingly, UNC-45 localization is also not seen in MHC B mutants, in which the level of MHC A is increased, resulting in near-normal muscle thick filament structure. Thus, filament assembly can be independent of UNC-45. UNC-45 shows a localization pattern identical to and dependent on MHC B and a function that appears to be MHC B-dependent. We propose that UNC-45 is a peripheral component of muscle thick filaments due to its localization with MHC B. The role of UNC-45 in thick filament assembly seems restricted to a cofactor for assembly or stabilization of MHC B.
Figures





References
-
- Ausubel, F.M., R. Brent, R.E. Kingston, D.D. Moore, J.A. Smith, J.G. Seidman, and K. Struh, editors. 1991. Current Protocols in Molecular Biology. John Wiley and Sons, Inc., New York. 1,600 pp.
-
- Bejsovec A., Anderson R.P. Myosin heavy chain mutations that disrupt C. elegans thick filament assembly. Genes Dev. 1988;2:1307–1317 . - PubMed
-
- Berteaux-Lecellier V., Zickler D., Debuchy R., Panvier-Adoutte A., Thompson-Coffe C., Picard M. A homologue of the yeast SHE4 gene is essential for the transition between the syncytial and cellular stages during sexual reproduction of the fungus Podospora anserina . EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:1248–1258 . - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

