Translation of chloroplast psbA mRNA is modulated in the light by counteracting oxidizing and reducing activities
- PMID: 10648596
- PMCID: PMC85229
- DOI: 10.1128/MCB.20.4.1116-1123.2000
Translation of chloroplast psbA mRNA is modulated in the light by counteracting oxidizing and reducing activities
Abstract
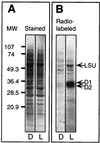
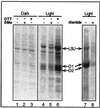
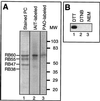
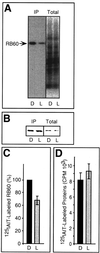
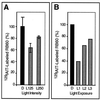
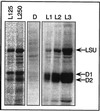
Light has been proposed to stimulate the translation of Chlamydomonas reinhardtii chloroplast psbA mRNA by activating a protein complex associated with the 5' untranslated region of this mRNA. The protein complex contains a redox-active regulatory site responsive to thioredoxin. We identified RB60, a protein disulfide isomerase-like member of the protein complex, as carrying the redox-active regulatory site composed of vicinal dithiol. We assayed in parallel the redox state of RB60 and translation of psbA mRNA in intact chloroplasts. Light activated the specific oxidation of RB60, on the one hand, and reduced RB60, probably via the ferredoxin-thioredoxin system, on the other. Higher light intensities increased the pool of reduced RB60 and the rate of psbA mRNA translation, suggesting that a counterbalanced action of reducing and oxidizing activities modulates the translation of psbA mRNA in parallel with fluctuating light intensities. In the dark, chemical reduction of the vicinal dithiol site did not activate translation. These results suggest a mechanism by which light primes redox-regulated translation by an unknown mechanism and then the rate of translation is determined by the reduction-oxidation of a sensor protein located in a complex bound to the 5' untranslated region of the chloroplast mRNA.
Figures


References
-
- Barber J, Anderson B. Too much of a good thing: light can be bad for photosynthesis. Trends Biochem Sci. 1992;17:61–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
