Identification of a novel element required for processing of intron-encoded box C/D small nucleolar RNAs in Saccharomyces cerevisiae
- PMID: 10648617
- PMCID: PMC85272
- DOI: 10.1128/MCB.20.4.1311-1320.2000
Identification of a novel element required for processing of intron-encoded box C/D small nucleolar RNAs in Saccharomyces cerevisiae
Abstract
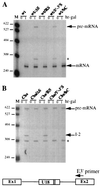
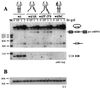
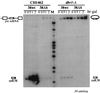
Processing of intron-encoded box C/D small nucleolar RNAs (snoRNAs) in metazoans through both the splicing-dependent and -independent pathways requires the conserved core motif formed by boxes C and D and the adjoining 5'-3'-terminal stem. By comparative analysis, we found that five out of six intron-encoded box C/D snoRNAs in yeast do not possess a canonical terminal stem. Instead, complementary regions within the flanking host intron sequences have been identified in all these cases. Here we show that these sequences are essential for processing of U18 and snR38 snoRNAs and that they compensate for the lack of a canonical terminal stem. We also show that the Rnt1p endonuclease, previously shown to be required for the processing of many snoRNAs encoded by monocistronic or polycistronic transcriptional units, is not required for U18 processing. Our results suggest a role of the complementary sequences in the early recognition of intronic snoRNA substrates and point out the importance of base pairing in favoring the communication between boxes C and D at the level of pre-snoRNA molecules for efficient assembly with snoRNP-specific factors.
Figures



References
-
- Amberg D C, Goldstein A L, Cole C N. Isolation and characterization of RAT1: an essential gene of Saccharomyces cerevisiae required for the efficient nucleocytoplasmic trafficking of mRNA. Genes Dev. 1992;6:1173–1189. - PubMed
-
- Bachellerie J-P, Michot B, Nicoloso M, Balakin A, Ni J, Fournier M J. Antisense snoRNAs: a family of nucleolar RNAs with long complementarities to rRNA. Trends Biochem Sci. 1995;20:261–264. - PubMed
-
- Bachellerie J-P, Cavaillé J. Guiding ribose methylation of rRNA. Trends Biochem Sci. 1997;22:257–261. - PubMed
-
- Balakin A G, Smith L, Fournier M J. The RNA world of the nucleolus: two major families of small RNAs defined by different box elements with related functions. Cell. 1996;86:823–834. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
