Regulatory interactions between the Reg1-Glc7 protein phosphatase and the Snf1 protein kinase
- PMID: 10648618
- PMCID: PMC85274
- DOI: 10.1128/MCB.20.4.1321-1328.2000
Regulatory interactions between the Reg1-Glc7 protein phosphatase and the Snf1 protein kinase
Abstract
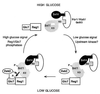
Protein phosphatase 1, comprising the regulatory subunit Reg1 and the catalytic subunit Glc7, has a role in glucose repression in Saccharomyces cerevisiae. Previous studies showed that Reg1 regulates the Snf1 protein kinase in response to glucose. Here, we explore the functional relationships between Reg1, Glc7, and Snf1. We show that different sequences of Reg1 interact with Glc7 and Snf1. We use a mutant Reg1 altered in the Glc7-binding motif to demonstrate that Reg1 facilitates the return of the activated Snf1 kinase complex to the autoinhibited state by targeting Glc7 to the complex. Genetic evidence indicated that the catalytic activity of Snf1 negatively regulates its interaction with Reg1. We show that Reg1 is phosphorylated in response to glucose limitation and that this phosphorylation requires Snf1; moreover, Reg1 is dephosphorylated by Glc7 when glucose is added. Finally, we show that hexokinase PII (Hxk2) has a role in regulating the phosphorylation state of Reg1, which may account for the effect of Hxk2 on Snf1 function. These findings suggest that the phosphorylation of Reg1 by Snf1 is required for the release of Reg1-Glc7 from the kinase complex and also stimulates the activity of Glc7 in promoting closure of the complex.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
