Secondary nicotinic synapses on sympathetic B neurons and their putative role in ganglionic amplification of activity
- PMID: 10648695
- PMCID: PMC6774159
- DOI: 10.1523/JNEUROSCI.20-03-00908.2000
Secondary nicotinic synapses on sympathetic B neurons and their putative role in ganglionic amplification of activity
Abstract
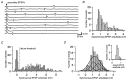
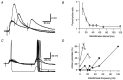
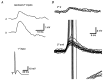
The strength and number of nicotinic synapses that converge on secretomotor B neurons were assessed in the bullfrog by recording intracellularly from isolated preparations of paravertebral sympathetic ganglia 9 and 10. One input to every B neuron invariably produced a suprathreshold EPSP and was defined as the primary nicotinic synapse. In addition, 93% of the cells received one to four subthreshold inputs that were defined as secondary nicotinic synapses. This contradicts the prevailing view, which has long held that amphibian B neurons are singly innervated. More important, the results revealed that B cells provide the simplest possible experimental system for examining the role of secondary nicotinic synapses on sympathetic neurons. Combining the convergence data with previous estimates of divergence indicates that the average preganglionic B neuron forms connections with 50 ganglionic B neurons and that the majority of these nicotinic synapses are secondary in strength. Secondary EPSPs evoked by low-frequency stimulation ranged from 0.5 to 10 mV in amplitude and had an average quantal content of 1. Nonetheless, secondary synapses could trigger action potentials via four mechanisms: spontaneous fluctuations of EPSP amplitude, two-pulse facilitation, coactivation with other secondary synapses, and coactivation with a slow peptidergic EPSP. The data were used to formulate a stochastic theory of integration, which predicts that ganglia function as amplifiers of the sympathetic outflow. In this two-component scheme, primary nicotinic synapses mediate invariant synaptic gain, and secondary nicotinic synapses mediate activity-dependent synaptic gain. The model also provides a common framework for considering how facilitation, metabotropic mechanisms, and preganglionic oscillators regulate synaptic amplification in sympathetic ganglia.
Figures






Similar articles
-
Excitatory muscarinic modulation strengthens virtual nicotinic synapses on sympathetic neurons and thereby enhances synaptic gain.J Neurophysiol. 2006 Dec;96(6):3104-13. doi: 10.1152/jn.00589.2006. Epub 2006 Sep 27. J Neurophysiol. 2006. PMID: 17005615 Free PMC article.
-
Presynaptic inhibition of cholinergic transmission by peptidergic neurons in bullfrog sympathetic ganglia.Pflugers Arch. 1988 Dec;413(2):206-8. doi: 10.1007/BF00582532. Pflugers Arch. 1988. PMID: 2851131
-
A presynaptic mechanism accounts for the differential block of nicotinic synapses on sympathetic B and C neurons by d-tubocurarine.J Neurosci. 1995 Jul;15(7 Pt 1):5025-35. doi: 10.1523/JNEUROSCI.15-07-05025.1995. J Neurosci. 1995. PMID: 7623131 Free PMC article.
-
The integrative role of synaptic cotransmission in the bullfrog vasomotor C system: evidence for a synaptic gain hypothesis.Can J Physiol Pharmacol. 1992;70 Suppl:S19-26. doi: 10.1139/y92-239. Can J Physiol Pharmacol. 1992. PMID: 1338294 Review.
-
Transmission of signals through sympathetic ganglia--modulation, integration or simply distribution?Acta Physiol Scand. 2003 Mar;177(3):227-35. doi: 10.1046/j.1365-201X.2003.01075.x. Acta Physiol Scand. 2003. PMID: 12608993 Review.
Cited by
-
The role of the paravertebral ganglia in human sympathetic neural discharge patterns.J Physiol. 2018 Sep;596(18):4497-4510. doi: 10.1113/JP276440. Epub 2018 Aug 19. J Physiol. 2018. PMID: 30054928 Free PMC article.
-
Vasomotor sympathetic neurons are more excitable than secretomotor sympathetic neurons in bullfrog paravertebral ganglia.Auton Neurosci. 2010 Jun 24;155(1-2):19-24. doi: 10.1016/j.autneu.2009.12.009. Epub 2010 Jan 27. Auton Neurosci. 2010. PMID: 20106724 Free PMC article.
-
Ganglionic transmission in a vasomotor pathway studied in vivo.J Physiol. 2010 May 1;588(Pt 9):1647-59. doi: 10.1113/jphysiol.2009.185025. Epub 2010 Mar 22. J Physiol. 2010. PMID: 20308254 Free PMC article.
-
Excitatory muscarinic modulation strengthens virtual nicotinic synapses on sympathetic neurons and thereby enhances synaptic gain.J Neurophysiol. 2006 Dec;96(6):3104-13. doi: 10.1152/jn.00589.2006. Epub 2006 Sep 27. J Neurophysiol. 2006. PMID: 17005615 Free PMC article.
-
Post-stimulus potentiation of transmission in pelvic ganglia enhances sympathetic dilatation of guinea-pig uterine artery in vitro.J Physiol. 2005 Jul 1;566(Pt 1):189-203. doi: 10.1113/jphysiol.2005.083493. Epub 2005 Mar 31. J Physiol. 2005. PMID: 15802294 Free PMC article.
References
-
- Akasu T, Kojima M, Koketsu K. Luteinizing hormone-releasing hormone modulates nicotinic ACh-receptor sensitivity in amphibian cholinergic transmission. Brain Res. 1983;279:347–351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
