Somatostatin modulates voltage-gated K(+) and Ca(2+) currents in rod and cone photoreceptors of the salamander retina
- PMID: 10648697
- PMCID: PMC3696031
- DOI: 10.1523/JNEUROSCI.20-03-00929.2000
Somatostatin modulates voltage-gated K(+) and Ca(2+) currents in rod and cone photoreceptors of the salamander retina
Abstract
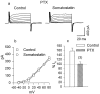
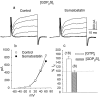
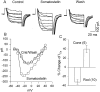
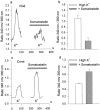
We investigated the cellular localization in the salamander retina of one of the somatostatin [or somatotropin release-inhibiting factor (SRIF)] receptors, sst(2A), and studied the modulatory action of SRIF on voltage-gated K(+) and Ca(2+) currents in rod and cone photoreceptors. SRIF immunostaining was observed in widely spaced amacrine cells, whose perikarya are at the border of the inner nuclear layer and inner plexiform layer. sst(2A) immunostaining was seen in the inner segments and terminals of rod and cone photoreceptors. Additional sst(2A) immunoreactivity was expressed by presumed bipolar and amacrine cells. SRIF, at concentrations of 100-500 nM, enhanced a delayed outwardly rectifying K(+) current (I(K)) in both rod and cone photoreceptors. SRIF action was blocked in cells pretreated with pertussis toxin (PTX) and was substantially reduced by intracellular GDP(beta)S. Voltage-gated L-type Ca(2+) currents in rods and cones were differently modulated by SRIF. SRIF reduced Ca(2+) current in rods by 33% but increased it in cones by 40%, on average. Both effects were mediated via G-protein activation and blocked by PTX. Ca(2+)-imaging experiments supported these results by showing that 500 nM SRIF reduced a K(+)-induced increase in intracellular Ca(2+) in rod photoreceptor terminals but increased it in those of cones. Our results suggest that SRIF may play a role in the regulation of glutamate transmitter release from photoreceptors via modulation of voltage-gated K(+) and Ca(2+) currents.
Figures



References
-
- Akopian A, Witkovsky P. D2 dopamine receptor-mediated inhibition of a hyperpolarization-activated current in rod photoreceptors. J Neurophysiol. 1996;76:1828–1835. - PubMed
-
- Attwell D. The photoreceptor output synapse. Prog Retin Res. 1990;9:337–362.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
