Kinetic differences between synaptic and extrasynaptic GABA(A) receptors in CA1 pyramidal cells
- PMID: 10648698
- PMCID: PMC6774173
- DOI: 10.1523/JNEUROSCI.20-03-00937.2000
Kinetic differences between synaptic and extrasynaptic GABA(A) receptors in CA1 pyramidal cells
Abstract
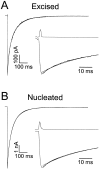
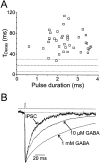
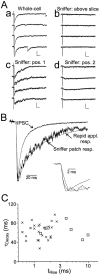
GABA(A)-mediated IPSCs typically decay more rapidly than receptors in excised patches in response to brief pulses of applied GABA. We have investigated the source of this discrepancy in CA1 pyramidal neurons. IPSCs in these cells decayed rapidly, with a weighted time constant tau(Decay) of approximately 18 msec (24 degrees C), whereas excised and nucleated patch responses to brief pulses of GABA (2 msec, 1 mM) decayed more than three times as slowly (tau(Decay), approximately 63 msec). This discrepancy was not caused by differences between synaptic and exogenous transmitter transients because (1) there was no dependence of tau(Decay) on pulse duration for pulses of 0.6-4 msec, (2) responses to GABA at concentrations as low as 10 microM were still slower to decay (tau(Decay), approximately 41 msec) than IPSCs, and (3) responses of excised patches to synaptically released GABA had decay times similar to brief pulse responses. These data indicate that the receptors mediating synaptic versus brief pulse responses have different intrinsic properties. However, synaptic receptors were not altered by the patch excision process, because fast, spontaneous IPSCs could still be recorded in nucleated patches. Elevated calcium selectively modulated patch responses to GABA pulses, with no effect on IPSCs recorded in nucleated patches, demonstrating the presence of two receptor populations that are differentially regulated by intracellular second messengers. We conclude that two receptor populations with distinct kinetics coexist in CA1 pyramidal cells: slow extrasynaptic receptors that dominate the responses of excised patches to exogenous GABA applications and fast synaptic receptors that generate rapid IPSCs.
Figures




References
-
- Assaf SY, Chung SH. Release of endogenous Zn 2+ from brain tissue during activity. Nature. 1984;308:734–736. - PubMed
-
- Banks MI, Pearce RA. Dual actions of volatile anesthetics on GABAA IPSCs: dissociation of blocking and prolonging effects. Anesthesiology. 1999;90:120–134. - PubMed
-
- Banks MI, Li T-B, Pearce RA. Effects of isoflurane on mIPSCs and excised neuronal GABAA receptors. Soc Neurosci Abstr. 1997;23:104.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
