A catalytic antioxidant metalloporphyrin blocks hydrogen peroxide-induced mitochondrial DNA damage
- PMID: 10648790
- PMCID: PMC102572
- DOI: 10.1093/nar/28.4.968
A catalytic antioxidant metalloporphyrin blocks hydrogen peroxide-induced mitochondrial DNA damage
Abstract
Reactive oxygen species (ROS) have been implicated as the cause of cumulative damage to DNA, proteins and lipids that can ultimately result in cell death. A common problem when measuring oxidative DNA damage has been the introduction of modifications in the native state of the molecule by many DNA isolation methods. We circumvented this problem by employing direct PCR (DPCR) of whole cell lysates. DPCR of mouse lung fibroblasts performed better than PCRs containing template acquired by phenol/chloroform extraction or a commercially available genomic DNA isolation kit. We investigated the direct use of whole cell preparations in the polymerase chain reaction (PCR) to detect hydrogen peroxide (H(2)O(2))-mediated DNA damage. We observed a concentration-dependent decrease in amplification efficiency of a 4.3 kb mitochondrial (mt)DNA target in H(2)O(2)-treated mouse lung fibroblasts (MLFs). At low doses the efficiency of amplification returns to control levels over 24 h. We detected no change in amplification efficiency of a plasmid control containing our mtDNA target under any of the culture conditions employed in these studies. Treatment of MLFs with the catalytic antioxidant manganese(III) meso -tetrakis(4-benzoic acid)porphyrin (MnTBAP) attenuates the effects of H(2)O(2)exposure. When quantitated with an external standard the use of DPCR in tandem with a PCR amplification efficiency assay provides a powerful approach to assess oxidative mtDNA damage.
Figures


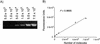

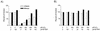
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

