Voltage- and tension-dependent lipid mobility in the outer hair cell plasma membrane
- PMID: 10650000
- PMCID: PMC1976274
- DOI: 10.1126/science.287.5453.658
Voltage- and tension-dependent lipid mobility in the outer hair cell plasma membrane
Abstract
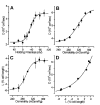
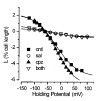
The mechanism responsible for electromotility of outer hair cells in the ear is unknown but is thought to reside within the plasma membrane. Lipid lateral diffusion in the outer hair cell plasma membrane is a sigmoidal function of transmembrane potential and bathing media osmolality. Cell depolarization or hyposmotic challenge shorten the cell and reduce membrane fluidity by half. Changing the membrane tension with amphipathic drugs results in similar reductions. These dynamic changes in membrane fluidity represent the modulation of membrane tension by lipid-protein interactions. The voltage dependence may be associated with the force-generating motors that contribute to the exquisite sensitivity of mammalian hearing.
Figures


References
-
- Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y. Science. 1985;227:194. - PubMed
-
- Vaz WLC, Derzko ZI, Jacobson KA. In: Membrane Reconstitution. Poste G, Nicolson GL, editors. Elsevier; Amsterdam: 1982. pp. 83–136.
-
- Golan DE, Alecio MR, Veatch WR, Rando RR. Biochemistry. 1984;23:332. - PubMed
-
-
The organ of Corti was isolated from the cochleae of guinea pigs, incubated for 3 to 5 min with trypsin, and dissociated onto a microwell petri dish. For some experiments, rat hippocampal neurons were used as a control. These cells were harvested on embryonic day 19 and grown in culture for 9 to 12 days in serum-free medium. Dissection and experiments were performed at 22°C in an extracellular solution consisting of the following: 155 mM NaCl, 4 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM Hepes, and 10 mM glucose. The solution was adjusted to a pH of 7.3 and an osmolality of 320 mOsm/kg. We stained the plasma membrane of the isolated cells with di-8-ANEPPS, a fluorescent molecule with a short hydrophobic tail that inserts itself into the outer leaflet of the phospholipid bilayer. Di-8-ANEPPS (75 μM) was dispersed into extracellular solution by sonication for 1 min, and the cells were stained for 5 min before unbound dye was thoroughly rinsed away with extracellular solution alone Montana V, Farkas DL, Loew LM. Biochemistry. 1989;28:4536.Oghalai JS, Tran TD, Raphael RM, Nakagawa T, Brownell WE. Hear Res. 1999;135:19.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

