Graft-versus-host-disease-associated donor cell engraftment in an F1 hybrid model is dependent upon the Fas pathway
- PMID: 10651946
- PMCID: PMC2327118
- DOI: 10.1046/j.1365-2567.2000.00919.x
Graft-versus-host-disease-associated donor cell engraftment in an F1 hybrid model is dependent upon the Fas pathway
Abstract
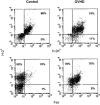
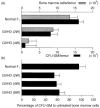
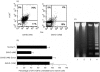
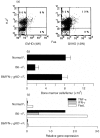
The graft-versus-host disease (GVHD) generated in BDF1 mice by the injection of spleen cells from the C57BL/6 parental strain induces a direct cell-mediated attack on host lymphohaematopoietic populations, resulting in the reconstitution of the host with donor cells. We examined Fas-Fas ligand (FasL) interactions in donor and host haematopoietic cells over a prolonged period of parental-induced GVHD. Fas expression on bone marrow cells of both donor and host origin increased at 2 weeks. Host cell incubation with anti-Fas antibody induced apoptosis, and the number of haematopoietic progenitor cells decreased. Fas-induced apoptosis by the repopulating donor cells, however, did not increase until 12 weeks, when more than 90% of the cells were donor cells. The expression of various cytokines, such as interferon-gamma (IFN-gamma) and tumour necrosis factor-alpha (TNF-alpha), and FasL gene expression in the bone marrow increased concomitantly. To examine directly whether FasL has a major role in the development of donor cell engraftment, FasL-deficient (gld) mice were used as donors. Injection of B6/gld spleen cells induced significantly less host lymphohaematopoietic depletion, resulting in a failure of donor cell engraftment. Furthermore, injection of IFN-gamma gene knockout (gko) B6 spleen cells failed to augment Fas and FasL expression in recipient mice, resulting in a failure of donor cell engraftment. This suggests that the induction of apoptosis by Fas-FasL interactions in host cells may contribute to a reconstitution of the host with donor cells and that donor-derived IFN-gamma plays a significant role for Fas-FasL interactions in host cells during parental-induced GVHD.
Figures

References
-
- Hakim FT, Shearer GM. Abrogation of hybrid resistence to bone marrow engraftment by graft‐vs‐host‐induced immune deficiency. J Immunol. 1986;137:3109. - PubMed
-
- Rolink AG, Radaszkiewicz T, Pals ST, van der Meer WG, Gleichmann E. Allosuppressor and allohelper T cells in acute and chronic graft‐vs‐host disease. I. Alloreactive suppressor cells rather than killer T cells appear to be the decisive effector cells in lethal graft‐vs‐host disease. J Exp Med. 1982;155:1502. - PMC - PubMed
-
- Iwasaki T, Fujiwara H, Iwasaki T, Shearer GM. Loss of proliferative capacity and T cell immune development potential by bone marrow from mice undergoing a graft‐vs‐host reaction. J Immunol. 1986;137:3100. - PubMed
-
- Hakim FT, Sharrow SO, Payne S, Sheare GM. Repopulation of host lymphohematopoietic systems by donor cells during graft‐versus‐host reaction in unirradiated adult F1 mice injected with parental lymphocytes. J Immunol. 1991;146:2108. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

