The regulation of prostaglandin output from term intact fetal membranes by anti-inflammatory cytokines
- PMID: 10651950
- PMCID: PMC2327135
- DOI: 10.1046/j.1365-2567.2000.00942.x
The regulation of prostaglandin output from term intact fetal membranes by anti-inflammatory cytokines
Abstract
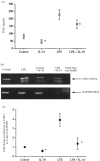
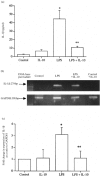
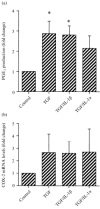
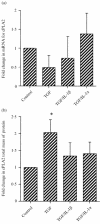
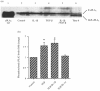
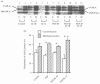
Prostaglandins are some of the main mediators which control parturition, and their production by intrauterine tissues can be up-regulated by pro-inflammatory cytokines. Anti-inflammatory cytokines may oppose these effects, and in this study we have investigated how two such cytokines affected fetal membrane function. Interleukin-10 (IL-10) inhibited the output of prostaglandin E2 (PGE2) from intact fetal membranes under basal and lipopolysaccharide (LPS)-stimulated conditions, and there was a parallel decrease in the expression of mRNA for COX-2. IL-10 also inhibited the production of interleukin-1beta (IL-1beta) and the expression of mRNA for IL-1beta, indicating that this cytokine has a broad anti-inflammatory effect. Transforming growth factor-beta1 (TGF-beta1), which is generally considered to be anti-inflammatory had opposite effects on PGE2 production, in that it increased the output of PGE2 for up to 8 hr. TGF-beta1 increased levels of type-2 cyclo-oxygenase (COX-2) and cytosolic phospholipase A2 (cPLA2) protein, and also activated the cPLA2 enzyme present; the profile of effects is similar to that of the pro-inflammatory cytokine IL-1beta, and was not expected. Combinations of TGF-beta1 with IL-1beta also increased PGE2 output and caused appropriate changes in prostaglandin pathway enzymes, whereas TGF-beta1 and IL-1alpha had more limited effects. Further studies are needed to establish the physiological significance of these findings, but TGF-beta1 does not seem to act as an inhibitory cytokine in intact fetal membranes at term.
Figures

Similar articles
-
Regulation of prostaglandin production in intact fetal membranes by interleukin-1 and its receptor antagonist.J Endocrinol. 1998 Dec;159(3):519-26. doi: 10.1677/joe.0.1590519. J Endocrinol. 1998. PMID: 9834469
-
The production of interleukin-1beta from human fetal membranes is not obligatory for increased prostaglandin output.Immunology. 1999 Jun;97(2):249-56. doi: 10.1046/j.1365-2567.1999.00769.x. Immunology. 1999. PMID: 10447739 Free PMC article.
-
Regulation of the cellular expression of secretory and cytosolic phospholipases A2, and cyclooxygenase-2 by peptide growth factors.Biochim Biophys Acta. 1998 May 27;1403(1):47-56. doi: 10.1016/s0167-4889(98)00029-9. Biochim Biophys Acta. 1998. PMID: 9622592
-
The regulation of cyclooxygenase-1 and -2 in knockout cells and cyclooxygenase and fever in knockout mice.Ernst Schering Res Found Workshop. 2000;(31):97-124. doi: 10.1007/978-3-662-04047-8_6. Ernst Schering Res Found Workshop. 2000. PMID: 10943330 Review. No abstract available.
-
Expression and function of phospholipase A(2) in brain.FEBS Lett. 2002 Oct 30;531(1):12-7. doi: 10.1016/s0014-5793(02)03481-6. FEBS Lett. 2002. PMID: 12401195 Review.
Cited by
-
IL-10 modulates placental responses to TLR ligands.Am J Reprod Immunol. 2009 Dec;62(6):390-9. doi: 10.1111/j.1600-0897.2009.00756.x. Epub 2009 Oct 11. Am J Reprod Immunol. 2009. PMID: 19821803 Free PMC article.
-
Tissue-specific cytokine release from human extra-placental membranes stimulated by lipopolysaccharide in a two-compartment tissue culture system.Reprod Biol Endocrinol. 2009 Oct 26;7:117. doi: 10.1186/1477-7827-7-117. Reprod Biol Endocrinol. 2009. PMID: 19857262 Free PMC article.
-
Mucosal tolerance to a bacterial superantigen indicates a novel pathway to prevent toxic shock.Infect Immun. 2002 May;70(5):2282-7. doi: 10.1128/IAI.70.5.2282-2287.2002. Infect Immun. 2002. PMID: 11953361 Free PMC article.
-
Preterm Cervical Ripening in humans.Facts Views Vis Obgyn. 2012;4(4):245-53. Facts Views Vis Obgyn. 2012. PMID: 24753916 Free PMC article. Review.
-
Preterm labor is characterized by a high abundance of amniotic fluid prostaglandins in patients with intra-amniotic infection or sterile intra-amniotic inflammation.J Matern Fetal Neonatal Med. 2021 Dec;34(24):4009-4024. doi: 10.1080/14767058.2019.1702953. Epub 2019 Dec 29. J Matern Fetal Neonatal Med. 2021. PMID: 31885290 Free PMC article.
References
-
- Duchesne MJ, Thaler‐dao H, Crastes de Paulet A. Prostaglandin synthesis in human placenta and fetal membranes. Prostaglandins. 1978;15:19. - PubMed
-
- Olson DM, Zakar T. Intrauterine tissue prostaglandin synthesis: Regulatory mechanisms. Semin Reprod Endocrinol. 1993;11:234.
-
- Nieder J, Augustin W. Increase of prostaglandin E and F equivalents in amniotic fluid during late pregnancy and rapid PGF elevation after cervical dilation. Prost Leukotr Med. 1983;12:289. - PubMed
-
- Romero R, Emamian M, Wan M, Qunitero R, Hobbins JC, Mitchell MD. Prostaglandin concentrations in amniotic fluid of women with intra‐amniotic infection and preterm labor. Am J Obstet Gynecol. 1988;157:1461. - PubMed
-
- Romero R, Baumann P, Gonzalez R, et al. Amniotic fluid prostanoid concentrations increase early during the course of spontaneous labour at term. Am J Obstet Gynecol. 1994;171:1613. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

