The gills are an important site of iNOS expression in rainbow trout Oncorhynchus mykiss after challenge with the gram-positive pathogen Renibacterium salmoninarum
- PMID: 10651954
- PMCID: PMC2327120
- DOI: 10.1046/j.1365-2567.2000.00914.x
The gills are an important site of iNOS expression in rainbow trout Oncorhynchus mykiss after challenge with the gram-positive pathogen Renibacterium salmoninarum
Abstract
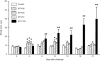
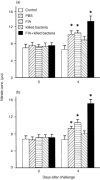
Following injection challenge of rainbow trout with the Gram-positive pathogen Renibacterium salmoninarum, serum nitrate levels increased indicative of NO production. The timing and amount of nitrate produced varied with the virulence of the bacterial strain used, with the highest levels seen in fish challenged with the most virulent (autoaggregating) strain. Immunization with a killed R. salmoninarum preparation in Freund's incomplete adjuvant significantly increased nitrate levels after challenge. Inducible nitric oxide synthase (iNOS) transcript expression was detectable in rainbow trout tissues after injection challenge with R. salmoninarum, and its induction in the gills was both quick (between 3 and 6 hr) and relatively prolonged (lasting several days). iNOS expression in the kidney was also seen at a later stage (24 hr) but appeared to switch off relatively rapidly. Bath challenge with R. salmoninarum also induced iNOS expression in gill, and a variable expression in the gut and kidney also occurred. These results highlight the importance of the gills, not only as a point of entry of pathogens but also as a tissue capable of mounting an immune response.
Figures




References
-
- Marletta MA, Yoon PS, Iyengar R, Leaf CD, Wishnok JS. Macrophage oxidation of l‐arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988;27:8706. - PubMed
-
- Clark IA, Rockett KA. Nitric oxide and parasitic disease. Adv Parasitol. 1996;37:1. - PubMed
-
- Bredt DS, Hwang PM, Glatt CE, Lowenstein C, Reed RR, Snyder SH. Cloned and expressed nitric oxide synthase structurally resembles cytochrome p‐450 reductase. Nature. 1991;351:714. - PubMed
-
- Bredt DS, Ferris CD, Snyder SH. Nitric oxide synthase regulatory sites: phosphorylation by cyclic AMP‐dependent protein kinase C, and calcium/calmodulin protein kinase; identification of flavin and calmodulin binding sites. J Biol Chem. 1992;267:10976. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

