A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle
- PMID: 10652278
- PMCID: PMC316354
A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle
Abstract
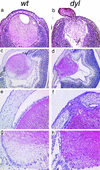
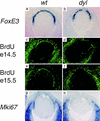
In the mouse mutant dysgenetic lens (dyl) the lens vesicle fails to separate from the ectoderm, causing a fusion between the lens and the cornea. Lack of a proliferating anterior lens epithelium leads to absence of secondary lens fibers and a dysplastic, cataractic lens. We report the cloning of a gene, FoxE3, encoding a forkhead/winged helix transcription factor, which is expressed in the developing lens from the start of lens placode induction and becomes restricted to the anterior proliferating cells when lens fiber differentiation begins. We show that FoxE3 is colocalized with dyl in the mouse genome, that dyl mice have mutations in the part of FoxE3 encoding the DNA-binding domain, and that these mutations cosegregate with the dyl phenotype. During embryonic development, the primordial lens epithelium is formed in an apparently normal way in dyl mutants. However, instead of the proliferation characteristic of a normal lens epithelium, the posterior of these cells fail to divide and show signs of premature differentiation, whereas the most anterior cells are eliminated by apoptosis. This implies that FoxE3 is essential for closure of the lens vesicle and is a factor that promotes survival and proliferation, while preventing differentiation, in the lens epithelium.
Figures






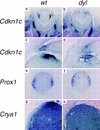

References
-
- Brahma SK, Sanyal S. Immunohistochemical studies of lens crystallins in the dysgenetic lens (dyl) mutant mice. Exp Eye Res. 1984;38:305–311. - PubMed
-
- ————— Ontogeny of alpha-crystallin polypeptides during the lens development of a mutant mouse. Curr Eye Res. 1987;6:1291–1297. - PubMed
-
- Brewitt B, Clark JI. Growth and transparency in the lens, an epithelial tissue, stimulated by pulses of PDGF. Science. 1988;242:777–779. - PubMed
-
- Chow RL, Roux GD, Roghani M, Palmer MA, Rifkin DB, Moscatelli DA, Lang RA. FGF suppresses apoptosis and induces differentiation of fibre cells in the mouse lens. Development. 1995;121:4383–4393. - PubMed
-
- Churchill AJ, Booth AP, Anwar R, Markham AF. PAX 6 is normal in most cases of Peters' anomaly. Eye. 1998;12:299–303. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
