Expression of the Staphylococcus hyicus lipase in Lactococcus lactis
- PMID: 10653722
- PMCID: PMC91867
- DOI: 10.1128/AEM.66.2.588-598.2000
Expression of the Staphylococcus hyicus lipase in Lactococcus lactis
Abstract
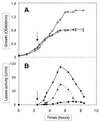
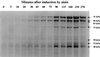
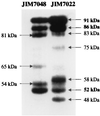
The extracellular Staphylococcus hyicus lipase was expressed under the control of different promoters in Lactococcus lactis and Bacillus subtilis. Its expression at high and moderate levels is toxic for the former and the latter hosts, respectively. In L. lactis, the lipase was expressed at a high level, up to 30% of the total cellular proteins, under the control of the inducible promoter PnisA. About 80% of the lipase remained associated with the cells. Close to half of this amount remained associated with the inner side of the cytoplasmic membrane as unprocessed pre-pro-lipase. The other half was trapped by the cell wall and partially degraded at the N-terminal end. This result suggests that extracellular proteases degrade the lipase. Surprisingly, the kinetics and the pattern of lipase degradation were different in the two L. lactis subspecies, L. lactis subsp. cremoris and L. lactis subsp. lactis. The extracellular proteolytic systems that degrade lipase are thus different in these closely related subspecies. The incorrect export of the lipase is not due to an inappropriate leader peptide but may be due to an inefficiency of several steps of lipase secretion. We propose that (i) the S. hyicus lipase may require a special accessory system to be correctly exported or (ii) the kinetics of lipase synthesis may be a critical factor for proper folding.
Figures




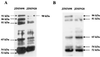
Similar articles
-
The peptidyl-prolyl isomerase motif is lacking in PmpA, the PrsA-like protein involved in the secretion machinery of Lactococcus lactis.Appl Environ Microbiol. 2002 Aug;68(8):3932-42. doi: 10.1128/AEM.68.8.3932-3942.2002. Appl Environ Microbiol. 2002. PMID: 12147493 Free PMC article.
-
Oral treatment with Lactococcus lactis expressing Staphylococcus hyicus lipase enhances lipid digestion in pigs with induced pancreatic insufficiency.Appl Environ Microbiol. 2002 Jun;68(6):3166-8. doi: 10.1128/AEM.68.6.3166-3168.2002. Appl Environ Microbiol. 2002. PMID: 12039786 Free PMC article.
-
Contributions of the pre- and pro-regions of a Staphylococcus hyicus lipase to secretion of a heterologous protein by Bacillus subtilis.Appl Environ Microbiol. 2010 Feb;76(3):659-69. doi: 10.1128/AEM.01671-09. Epub 2009 Nov 30. Appl Environ Microbiol. 2010. PMID: 19948853 Free PMC article.
-
Staphylococcal lipases: molecular characterisation, secretion, and processing.Chem Phys Lipids. 1998 Jun;93(1-2):15-25. doi: 10.1016/s0009-3084(98)00025-5. Chem Phys Lipids. 1998. PMID: 9720246 Review.
-
Lactococcus lactis, an efficient cell factory for recombinant protein production and secretion.J Mol Microbiol Biotechnol. 2008;14(1-3):48-58. doi: 10.1159/000106082. J Mol Microbiol Biotechnol. 2008. PMID: 17957110 Review.
Cited by
-
Factors Affecting Inducible Expression of Outer Membrane Protein A (OmpA) of Shigella dysenteriae Type-1 in Lactococcus lactis Using Nisin Inducible Controlled Expression (NICE).Indian J Microbiol. 2016 Mar;56(1):80-7. doi: 10.1007/s12088-015-0556-2. Epub 2015 Oct 27. Indian J Microbiol. 2016. PMID: 26843700 Free PMC article.
-
Shifting the pH profiles of Staphylococcus epidermidis lipase (SEL) and Staphylococcus hyicus lipase (SHL) through generating chimeric lipases by DNA shuffling strategy.World J Microbiol Biotechnol. 2024 Feb 22;40(4):106. doi: 10.1007/s11274-024-03927-x. World J Microbiol Biotechnol. 2024. PMID: 38386107
-
Enzyme replacement therapy for pancreatic insufficiency: present and future.Clin Exp Gastroenterol. 2011;4:55-73. doi: 10.2147/CEG.S17634. Epub 2011 May 4. Clin Exp Gastroenterol. 2011. PMID: 21753892 Free PMC article.
-
The peptidyl-prolyl isomerase motif is lacking in PmpA, the PrsA-like protein involved in the secretion machinery of Lactococcus lactis.Appl Environ Microbiol. 2002 Aug;68(8):3932-42. doi: 10.1128/AEM.68.8.3932-3942.2002. Appl Environ Microbiol. 2002. PMID: 12147493 Free PMC article.
-
Expression of an organic solvent stable lipase from Staphylococcus epidermidis AT2.Int J Mol Sci. 2010 Sep 13;11(9):3195-208. doi: 10.3390/ijms11093195. Int J Mol Sci. 2010. PMID: 20957088 Free PMC article.
References
-
- Anba J, Pages J M, Lazdunski C. Mode of transfer of the phosphate-binding protein through the cytoplasmic membrane in Escherichia coli. FEMS Microbiol Lett. 1986;34:215–219.
-
- Braun P, Tommassen J, Filloux A. Role of the propeptide in folding and secretion of elastase of Pseudomonas aeruginosa. Mol Microbiol. 1996;19:297–306. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

