Cotransformation of Trichoderma harzianum with beta-glucuronidase and green fluorescent protein genes provides a useful tool for monitoring fungal growth and activity in natural soils
- PMID: 10653755
- PMCID: PMC91900
- DOI: 10.1128/AEM.66.2.810-815.2000
Cotransformation of Trichoderma harzianum with beta-glucuronidase and green fluorescent protein genes provides a useful tool for monitoring fungal growth and activity in natural soils
Abstract
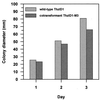
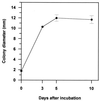
Trichoderma harzianum was cotransformed with genes encoding green fluorescent protein (GFP), beta-glucuronidase (GUS), and hygromycin B (hygB) resistance, using polyethylene glycol-mediated transformation. One cotransformant (ThzID1-M3) was mitotically stable for 6 months despite successive subculturing without selection pressure. ThzID1-M3 morphology was similar to that of the wild type; however, the mycelial growth rate on agar was reduced. ThzID1-M3 was formed into calcium alginate pellets and placed onto buried glass slides in a nonsterile soil, and its ability to grow, sporulate, and colonize sclerotia of Sclerotinia sclerotiorum was compared with that of the wild-type strain. Wild-type and transformant strains both colonized sclerotia at levels above those of indigenous Trichoderma spp. in untreated controls. There were no significant differences in colonization levels between wild-type and cotransformant strains; however, the presence of the GFP and GUS marker genes permitted differentiation of introduced Trichoderma from indigenous strains. GFP activity was a useful tool for nondestructive monitoring of the hyphal growth of the transformant in a natural soil. The green color of cotransformant hyphae was clearly visible with a UV epifluorescence microscope, while indigenous fungi in the same samples were barely visible. Green-fluorescing conidiophores and conidia were observed within the first 3 days of incubation in soil, and this was followed by the formation of terminal and intercalary chlamydospores and subsequent disintegration of older hyphal segments. Addition of 5-bromo-4-chloro-3-indolyl-beta-D-glucuronic acid (X-Gluc) substrate to recovered glass slides confirmed the activity of GUS as well as GFP in soil. Our results suggest that cotransformation with GFP and GUS can provide a valuable tool for the detection and monitoring of specific strains of T. harzianum released into the soil.
Figures



References
-
- Abd-El Moity T H, Papavizas G C, Shatla M N. Induction of new isolates of Trichoderma harzianum tolerant to fungicides and their experimental use for control of white rot of onion. Phytopathology. 1982;72:396–400.
-
- Ahmad J S, Baker R. Rhizosphere competence of Trichoderma harzianum. Phytopathology. 1987;77:182–189.
-
- Bin L, Knudsen G R, Eschen D J. Influence of an antagonistic strain of Pseudomonas fluorescens on growth and ability of Trichoderma harzianum to colonize sclerotia of Sclerotinia sclerotiorum in soil. Phytopathology. 1991;81:994–1000.
-
- Bollen G J, Middelkoop J, Hofman T W. Effects of soil fauna on infection of potato sprouts by Rhizoctonia solani. In: Beemster A B R, Bollen G J, Gerlagh M, Ruissen M A, Schippers B, Tempel A, editors. Biotic interactions and soil-borne diseases. Proceedings of the First Conference of the European Foundation for Plant Pathology. New York, N.Y: Elsevier Science Publishing Co., Inc.; 1991. pp. 27–34.
-
- Chalfie M, Tu Y, Euskirchen G, Ward W W, Prasher D C. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

