Mechanism of rescue of common p53 cancer mutations by second-site suppressor mutations
- PMID: 10654936
- PMCID: PMC305574
- DOI: 10.1093/emboj/19.3.370
Mechanism of rescue of common p53 cancer mutations by second-site suppressor mutations
Abstract
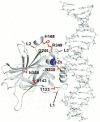
The core domain of p53 is extremely susceptible to mutations that lead to loss of function. We analysed the stability and DNA-binding activity of such mutants to understand the mechanism of second-site suppressor mutations. Double-mutant cycles show that N239Y and N268D act as 'global stability' suppressors by increasing the stability of the cancer mutants G245S and V143A-the free energy changes are additive. Conversely, the suppressor H168R is specific for the R249S mutation: despite destabilizing wild type, H168R has virtually no effect on the stability of R249S, but restores its binding affinity for the gadd45 promoter. NMR structural comparisons of R249S/H168R and R249S/T123A/H168R with wild type and R249S show that H168R reverts some of the structural changes induced by R249S. These results have implications for possible drug therapy to restore the function of tumorigenic mutants of p53: the function of mutants such as V143A and G245S is theoretically possible to restore by small molecules that simply bind to and hence stabilize the native structure, whereas R249S requires alteration of its mutant native structure.
Figures



References
-
- Buckle A.M., Henrick, K. and Fersht, A.R. (1993) Crystal structural analysis of mutations in the hydrophobic cores of barnase. J. Mol. Biol., 234, 847–860. - PubMed
-
- Carter P.G., Winter, G., Wilkinson, A.J. and Fersht, A.R. (1984) The use of double mutants to detect structural changes in the active site of the tyrosyl-tRNA synthetase (Bacillus stearothermophilus). Cell, 38, 835–840. - PubMed
-
- Cho Y., Gorina, S., Jeffrey, P.D. and Pavletich, N.P. (1994) Crystal structure of a p53 tumor suppressor–DNA complex: understanding tumorigenic mutations. Science, 265, 346–355. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

