High levels of Epstein-Barr virus DNA in blood of solid-organ transplant recipients and their value in predicting posttransplant lymphoproliferative disorders
- PMID: 10655355
- PMCID: PMC86158
- DOI: 10.1128/JCM.38.2.613-619.2000
High levels of Epstein-Barr virus DNA in blood of solid-organ transplant recipients and their value in predicting posttransplant lymphoproliferative disorders
Abstract
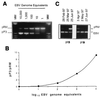
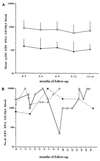
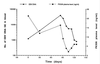
Epstein-Barr virus (EBV) DNA was quantitated in peripheral blood mononuclear cells (PBMC) from 25 healthy subjects, 105 asymptomatic solid-organ transplant (SOT) recipients, and 15 SOT recipients with symptomatic EBV infections by using a newly developed quantitative-PCR technique. Patients with symptomatic EBV infections had significantly higher (P < 0.001) median EBV DNA levels than asymptomatic SOT recipients and immunocompetent individuals. In SOT recipients, the positive predictive value of EBV DNA levels of >1, 000 genome equivalents (GE)/0.5 microg of total PBMC DNA was 64.7% for symptomatic EBV infection, while the negative predictive value was 96.1%. In 19 of 32 (59.3%) asymptomatic SOT recipients, EBV DNA levels were consistently below 1,000 GE for as long as 18 months, while 10 of 32 (31.2%) patients had 1,000 to 5,000 EBV GE at least once during follow-up. In a minority of patients (3 of 32; 9.3%), >/=5,000 GE could be detected at least once during follow-up. Reduction of immunosuppressive treatment decreased EBV DNA levels by >/=1 log(10) unit in patients with symptomatic EBV infections. Quantification of EBV DNA is valuable for the diagnosis and monitoring of symptomatic EBV infections in SOT recipients.
Figures


References
-
- Alfrey E J, Friedman A L, Grossman R A, Perloff L J, Naji A, Barker C F, Montone K T, Tomaszewski J E, Chmielewski C, Holland T, Zmijewski C, Dafoe D C. A recent decrease in the time to development of monomorphous and polymorphous post-transplant lymphoproliferative disorder. Transplantation. 1992;54:250–253. - PubMed
-
- Armitage J M, Kormos R L, Stuart R S, Fricker F J, Griffith B P, Nalesnik M, Hardesty R L, Dummer J S. Posttransplant lymphoproliferative disease in thoracic organ transplant patients: ten years of cyclosporine-based immunosuppression. J Heart Lung Transplant. 1991;10:877–886. - PubMed
-
- Cleary M L, Sklar J. Lymphoproliferative disorders in cardiac transplant recipients are multiclonal lymphomas. Lancet. 1984;2:489–493. - PubMed
-
- Craig F E, Gulley M L, Banks P M. Posttransplantation lymphoproliferative disorders. Am J Clin Pathol. 1993;99:265–276. - PubMed
-
- Fischer A, Blanche S, Le Bidois J, Bordigoni P, Garnier J L, Niaudet P, Morinet F, Le Deist F, Fischer A M, Griscelli C, Hirn M. Anti-B-cell monoclonal antibodies in the treatment of severe B-cell lymphoproliferative syndrome following bone marrow and organ transplantation. N Engl J Med. 1991;324:1451–1456. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

