Rational discovery of novel nuclear hormone receptor antagonists
- PMID: 10655475
- PMCID: PMC15503
- DOI: 10.1073/pnas.97.3.1008
Rational discovery of novel nuclear hormone receptor antagonists
Abstract
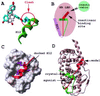
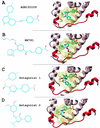
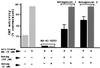
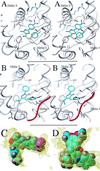
Nuclear hormone receptors (NRs) are potential targets for therapeutic approaches to many clinical conditions, including cancer, diabetes, and neurological diseases. The crystal structure of the ligand binding domain of agonist-bound NRs enables the design of compounds with agonist activity. However, with the exception of the human estrogen receptor-alpha, the lack of antagonist-bound "inactive" receptor structures hinders the rational design of receptor antagonists. In this study, we present a strategy for designing such antagonists. We constructed a model of the inactive conformation of human retinoic acid receptor-alpha by using information derived from antagonist-bound estrogen receptor-alpha and applied a computer-based virtual screening algorithm to identify retinoic acid receptor antagonists. Thus, the currently available crystal structures of NRs may be used for the rational design of antagonists, which could lead to the development of novel drugs for a variety of diseases.
Figures
References
-
- Dees E C, Kennedy M J. Curr Opin Oncol. 1998;10:517–522. - PubMed
-
- Fanjul A N, Piedrafita F J, Al-Shamma H, Pfahl M. Cancer Res. 1998;58:4607–4610. - PubMed
-
- Shiohara M, Dawson M I, Hobbs P D, Sawai N, Higuchi T, Koike K, Komiyama A, Koeffler H P. Blood. 1999;93:2057–2066. - PubMed
-
- Bischoff E D, Moon T E, Heyman R A, Lamph W W. Cancer Res. 1998;58:479–484. - PubMed
-
- Mukherjee R, Davies P J, Paterniti J R, Jr, Heyman R A. Nature (London) 1997;386:407–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

