The sentrin-conjugating enzyme mUbc9 interacts with GLUT4 and GLUT1 glucose transporters and regulates transporter levels in skeletal muscle cells
- PMID: 10655495
- PMCID: PMC15543
- DOI: 10.1073/pnas.97.3.1125
The sentrin-conjugating enzyme mUbc9 interacts with GLUT4 and GLUT1 glucose transporters and regulates transporter levels in skeletal muscle cells
Abstract
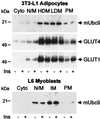
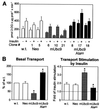
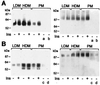
Glucose transport in insulin-regulated tissues is mediated by the GLUT4 and GLUT1 transporters. Using the yeast two-hybrid system, we have cloned the sentrin-conjugating enzyme mUbc9 as a protein that interacts with the GLUT4 COOH-terminal intracellular domain. The mUbc9 enzyme was found to bind directly to GLUT4 and GLUT1 through an 11-aa sequence common to the two transporters and to modify both transporters covalently by conjugation with the mUbc9 substrate, sentrin. Overexpression of mUbc9 in L6 skeletal muscle cells decreased GLUT1 transporter abundance 65%, resulting in decreased basal glucose transport. By contrast, mUbc9 overexpression increased GLUT4 abundance 8-fold, leading to enhanced transport stimulation by insulin. A dominant-negative mUbc9 mutant lacking catalytic activity had effects opposite to those of wild-type mUbc9. The regulation of GLUT4 and GLUT1 was specific, as evidenced by an absence of mUbc9 interaction with or regulation of the GLUT3 transporter isoform in L6 skeletal muscle cells. The mUbc9 sentrin-conjugating enzyme represents a novel regulator of GLUT1 and GLUT4 protein levels with potential importance as a determinant of basal and insulin-stimulated glucose uptake in normal and pathophysiological states.
Figures


References
-
- Bell G I, Burant C F, Takeda J, Gould G W. J Biol Chem. 1993;268:19161–19164. - PubMed
-
- Pessin J E, Bell G I. Annu Rev Physiol. 1992;54:911–930. - PubMed
-
- Piper R C, Hess L J, James D E. Am J Physiol. 1991;260:C570–C580. - PubMed
-
- Rodnick K J, Slot J W, Studelska D R, Hanpeter D E, Robinson L J, Geuze H J, James D E. J Biol Chem. 1992;267:6278–6285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

