Expression of guanylin in "pars tuberalis-specific cells" and gonadotrophs of rat adenohypophysis
- PMID: 10655496
- PMCID: PMC15545
- DOI: 10.1073/pnas.97.3.1131
Expression of guanylin in "pars tuberalis-specific cells" and gonadotrophs of rat adenohypophysis
Abstract
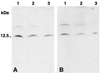
The intestinal peptide guanylin regulates the electrolyte/water transport in the gastrointestinal epithelium by paracrine/luminocrine mechanisms. Because guanylin also circulates in the blood, we investigated the rat hypothalamo-pituitary region for expression and cellular localization of this peptide. Reverse transcriptase-PCR analyses with guanylin-specific primers revealed expression of the peptide in the pars tuberalis and pars distalis of the pituitary. Western blotting analyses in hypophyseal tissue extracts identified the expected 12.5-kDa immunoreactive peptide by using two different region-specific guanylin antisera. Light and electron microscopic immunocytochemistry with the same antisera localized guanylin in "pars tuberalis-specific cells" in the juxtaneural pars tuberalis adjacent to nerve endings and blood vessels of the hypothalamo-pituitary portal system and in gonadotrophic cells within the distal pars tuberalis and ventrolateral part of the pars distalis. The presence and cell-specific localization of guanylin within the hypothalamo-hypophyseal system indicate that this peptide may be specifically involved in paracrine and endocrine regulatory mechanisms.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

