A mutation in the alpha 3 chain of type IX collagen causes autosomal dominant multiple epiphyseal dysplasia with mild myopathy
- PMID: 10655510
- PMCID: PMC15572
- DOI: 10.1073/pnas.97.3.1212
A mutation in the alpha 3 chain of type IX collagen causes autosomal dominant multiple epiphyseal dysplasia with mild myopathy
Abstract
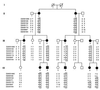
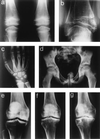
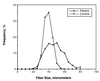
Multiple epiphyseal dysplasia (MED) is a degenerative cartilage condition shown in some cases to be caused by mutations in genes encoding cartilage oligomeric matrix protein or type IX collagen. We studied a family with autosomal dominant MED affecting predominantly the knee joints and a mild proximal myopathy. Genetic linkage to the COL9A3 locus on chromosome 20q13.3 was established with a peak log(10) odds ratio for linkage score of 3.87 for markers D20S93 and D20S164. Reverse transcription-PCR performed on the muscle biopsy revealed aberrant mRNA lacking exon 3, which predicted a protein lacking 12 amino acids from the COL3 domain of alpha3(IX) collagen. Direct sequencing of genomic DNA confirmed the presence of a splice acceptor mutation in intron 2 of the COL9A3 gene (intervening sequence 2, G-A, -1) only in affected family members. By electron microscopy, chondrocytes from epiphyseal cartilage exhibited dilated rough endoplasmic reticulum containing linear lamellae of alternating electron-dense and electron-lucent material, reflecting abnormal processing of mutant protein. Type IX collagen chains appeared normal in size and quantity but showed defective cross-linking by Western blotting. The novel phenotype of MED and mild myopathy is likely caused by a dominant-negative effect of the exon 3-skipping mutation in the COL9A3 gene. Patients with MED and a waddling gait but minimal radiographic hip involvement should be evaluated for a primary myopathy and a mutation in type IX collagen.
Figures


 ). (c)
Schematic of the splice defect relative to the domain structure of
COL9A3. COL1–3, collagenous domains 1–3; and NC1–4,
noncollagenous domains 1–4.
). (c)
Schematic of the splice defect relative to the domain structure of
COL9A3. COL1–3, collagenous domains 1–3; and NC1–4,
noncollagenous domains 1–4.
References
-
- Fairbank T. Br J Surg. 1947;34:225–232. - PubMed
-
- Rimoin D L, Rasmussen I M, Briggs M D, Roughly P J, Gruber H E, Warman M L, Olsen B R, Hsia Y E, Yuen J, Reinker K, et al. Hum Genet. 1994;93:236–242. - PubMed
-
- Ribbing S. Acta Radiol Suppl. 1937;34:1–107.
-
- International Working Group on Constitutional Disorders of Bone. Am J Med Genet. 1998;79:376–382. - PubMed
-
- Briggs M D, Hoffman S M, King L M, Olsen A S, Mohrenweiser H, Leroy J G, Mortier G R, Rimoin D L, Lachman R S, Gaines E S, et al. Nat Genet. 1995;10:330–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

