In situ detection of AP sites and DNA strand breaks bearing 3'-phosphate termini in ischemic mouse brain
- PMID: 10657997
- PMCID: PMC2746459
- DOI: 10.1096/fasebj.14.2.407
In situ detection of AP sites and DNA strand breaks bearing 3'-phosphate termini in ischemic mouse brain
Abstract
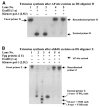
Our aims were to examine whether oxidative DNA damage was elevated in brain cells of male C57BL/6 mice after oxidative stress, and to determine whether neuronal nitric oxide synthase (nNOS) was involved in such damage. Oxidative stress was induced by occluding both common carotid arteries for 90 min, followed by reperfusion. Escherichia coli exonuclease III (Exo III) removes apyrimidinic or apurinic (AP) sites and 3'-phosphate termini in single-strand breaks, and converts these lesions to 3'OH termini. These ExoIII-sensitive sites (EXOSS) can then be postlabeled using digoxigenin-11-dUTP and Klenow DNA polymerase-I, and detected using fluorescein isothiocyanate-IgG against digoxigenin. Compared with the non-ischemia controls, the density of EXOSS-positive cells was elevated at least 20-fold (P < 0.01) at 15 min of reperfusion, and remained elevated for another 30 min. EXOSS mainly occurred in the cell nuclei of the astrocytes and neurons. Signs of cell death were detected at 24 h of reperfusion and occurred mostly in the neurons. Both DNA damage and cell death in the cerebral cortical neurons were abolished by treatment with 3-bromo-7-nitroindazole (30 mg/kg, intraperitoneal), which specifically inhibited nNOS. Our results suggest that nNOS, its activator (calcium), and peroxynitrite exacerbate oxidative DNA damage after brain ischemia.-Huang, D., Shenoy, A., Cui, J., Huang, W., Liu, P. In situ detection of AP sites and DNA strand breaks bearing 3'-phosphate termini in ischemic mouse brain.
Figures








Similar articles
-
In situ detection of neuronal DNA strand breaks using the Klenow fragment of DNA polymerase I reveals different mechanisms of neuron death after global cerebral ischemia.J Neurochem. 1999 Mar;72(3):1204-14. doi: 10.1046/j.1471-4159.1999.0721204.x. J Neurochem. 1999. PMID: 10037493
-
Induction of oxidative DNA damage in the peri-infarct region after permanent focal cerebral ischemia.J Neurochem. 2000 Oct;75(4):1716-28. doi: 10.1046/j.1471-4159.2000.0751716.x. J Neurochem. 2000. PMID: 10987855
-
Oxidative DNA damage precedes DNA fragmentation after experimental stroke in rat brain.FASEB J. 2000 May;14(7):955-67. doi: 10.1096/fasebj.14.7.955. FASEB J. 2000. PMID: 10783150 Free PMC article.
-
Oxidative stress in brain ischemia.Brain Pathol. 1999 Jan;9(1):119-31. doi: 10.1111/j.1750-3639.1999.tb00214.x. Brain Pathol. 1999. PMID: 9989455 Free PMC article. Review.
-
Ischemia-reperfusion-related repair deficit after oxidative stress: implications of faulty transcripts in neuronal sensitivity after brain injury.J Biomed Sci. 2003 Jan-Feb;10(1):4-13. doi: 10.1007/BF02255992. J Biomed Sci. 2003. PMID: 12566981 Free PMC article. Review.
Cited by
-
Cooling and Sterile Inflammation in an Oxygen-Glucose-Deprivation/Reperfusion Injury Model in BV-2 Microglia.Mediators Inflamm. 2021 Nov 5;2021:8906561. doi: 10.1155/2021/8906561. eCollection 2021. Mediators Inflamm. 2021. PMID: 34776788 Free PMC article.
-
Oxidative stress alters base excision repair pathway and increases apoptotic response in apurinic/apyrimidinic endonuclease 1/redox factor-1 haploinsufficient mice.Free Radic Biol Med. 2009 Jun 1;46(11):1488-99. doi: 10.1016/j.freeradbiomed.2009.02.021. Epub 2009 Mar 3. Free Radic Biol Med. 2009. PMID: 19268524 Free PMC article.
-
Tea polyphenols increase X-ray repair cross-complementing protein 1 and apurinic/apyrimidinic endonuclease/redox factor-1 expression in the hippocampus of rats during cerebral ischemia/reperfusion injury.Neural Regen Res. 2012 Oct 25;7(30):2355-61. doi: 10.3969/j.issn.1673-5374.2012.30.005. Neural Regen Res. 2012. PMID: 25538760 Free PMC article.
-
Noninvasive detection of neural progenitor cells in living brains by MRI.FASEB J. 2012 Apr;26(4):1652-62. doi: 10.1096/fj.11-199547. Epub 2011 Dec 23. FASEB J. 2012. PMID: 22198388 Free PMC article.
-
DNA polymerase beta-catalyzed-PCNA independent long patch base excision repair synthesis: a mechanism for repair of oxidatively damaged DNA ends in post-mitotic brain.J Neurochem. 2008 Nov;107(3):734-44. doi: 10.1111/j.1471-4159.2008.05644.x. Epub 2008 Sep 20. J Neurochem. 2008. PMID: 18752643 Free PMC article.
References
-
- Choi DW. Calcium: Still center-stage in hypoxicischemic neuronal death. Trends Neurosci. 1995;18:58–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

