Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons
- PMID: 10662830
- PMCID: PMC6772355
- DOI: 10.1523/JNEUROSCI.20-04-01393.2000
Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons
Abstract
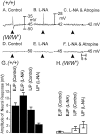
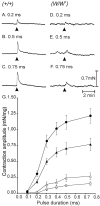
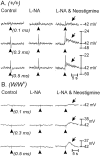
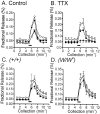
Interstitial cells of Cajal (ICC) are interposed between enteric neurons and smooth muscle cells in gastrointestinal muscles. The role of intramuscular ICC (IC-IM) in mediating enteric excitatory neural inputs was studied using gastric fundus muscles of wild-type animals and W/W(v) mutant mice, which lack IC-IM. Excitatory motor neurons, labeled with antibodies to vesicular acetylcholine transporter or substance-P, were closely associated with IC-IM. Immunocytochemistry showed close contacts between enteric neurons and IC-IM. IC-IM also formed gap junctions with smooth muscle cells. Electrical field stimulation yielded fast excitatory junction potentials in the smooth muscle that were blocked by atropine. Neural responses were greatly reduced in muscles of W/W(v) animals. Loss of cholinergic responses in W/W(v) muscles seemed to be caused by the loss of close synaptic contacts between motor neurons and IC-IM, because these muscles were not less responsive to exogenous acetylcholine than were wild-type muscles. W/W(v) muscles also responded to excitatory nerve stimulation when the breakdown of acetylcholine was blocked by neostigmine. The density of cholinergic nerve bundles within the muscles was not significantly different in wild-type and W/W(v) muscles, and similar amounts of (14)[C]choline were released from preloaded wild-type and W/W(v) muscles in response to nerve stimulation. The impact of losing IC-IM on gastric compliance was also evaluated in intact stomachs. Pressure increased as a function of fluid volume and infusion rate in wild-type animals, but W/W(v) animals showed little basal tone and minimal increases in pressure with fluid infusions. These data suggest that IC-IM play a major role in receiving cholinergic excitatory inputs from the enteric nervous system in the murine fundus.
Figures







References
-
- Daniel EE, Posey-Daniel V. Neuromuscular structures in opossum esophagus: role of interstitial cells of Cajal. Am J Physiol. 1984;246:G305–G315. - PubMed
-
- Faussone-Pellegrini MS, Cortesini C. The muscle coat of the lower esophageal sphincter in patients with achalasia and hypertensive sphincter. An electron microscopic study. J Submicrosc Cytol. 1985;17:673–685. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
