Consequences of neural cell adhesion molecule deficiency on cell migration in the rostral migratory stream of the mouse
- PMID: 10662835
- PMCID: PMC6772373
- DOI: 10.1523/JNEUROSCI.20-04-01446.2000
Consequences of neural cell adhesion molecule deficiency on cell migration in the rostral migratory stream of the mouse
Abstract
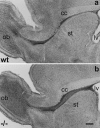
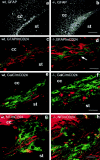
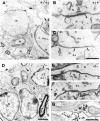
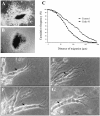
In vertebrates, interneurons of the olfactory bulb (OB) are generated postnatally and throughout life at the subventricular zone of the forebrain. The neuronal precursors migrate tangentially through the forebrain using a well defined pathway, the rostral migratory stream (RMS), and a particular mode of migration in a chain-like organization. A severe size reduction of the OB represents the most striking morphological phenotype in neural cell adhesion molecule (NCAM)-deficient mice. This defect has been traced back to a migration deficit of the precursors in the RMS and linked to the lack of the polysialylated form of NCAM. In this study we investigate the morphological alterations and functional properties of the RMS in mice totally devoid of all isoforms of NCAM and polysialic acid (PSA). We show that a morphologically altered, but defined and continuous pathway exists in mutants, and we present in vivo and in vitro evidence that PSA-NCAM in the RMS is not essential for the formation and migration of chains. Instead, we find a massive gliosis associated with the formation of membrane specializations in a heterotypic manner, linking precursors to astrocytes. This finding and the over-representation and defasciculation of axons in the pathway suggest that important interactions between migrating cells and their stationary environment are perturbed in the mutants. Finally, we used transplantation experiments to demonstrate that lack of PSA-NCAM leads to a decrease but not a total blockade of migration and demonstrate that the mutant RMS is functional in transporting normal neuronal precursors to the OB.
Figures





References
-
- Alvarez-Buylla A. Mechanism of migration of olfactory bulb interneurons. Cell Dev Biol. 1997;8:207–213. - PubMed
-
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–458. - PubMed
-
- Berryman MA, Rodewald RD. An enhanced method for post-embedding immunocytochemical staining which preserves cell membranes. J Histochem Cytochem. 1990;38:159–170. - PubMed
-
- Calaora V, Chazal G, Nielsen PJ, Rougon G, Moreau H. mCD24 expression in the developing mouse brain and in zones of secondary neurogenesis in the adult. Neuroscience. 1996;73:581–594. - PubMed
-
- Cohen-Tannoudji M, Morello D, Babinet C. Unexpected position-dependence of H-2 and β2 microglobulin/lacZ transgenes. Mol Reprod Dev. 1992;33:149–159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
