T-cell receptor-mediated anergy of a human immunodeficiency virus (HIV) gp120-specific CD4(+) cytotoxic T-cell clone, induced by a natural HIV type 1 variant peptide
- PMID: 10666241
- PMCID: PMC111692
- DOI: 10.1128/jvi.74.5.2121-2130.2000
T-cell receptor-mediated anergy of a human immunodeficiency virus (HIV) gp120-specific CD4(+) cytotoxic T-cell clone, induced by a natural HIV type 1 variant peptide
Abstract
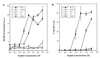
Human immunodeficiency virus type 1 (HIV-1) infection triggers a cytotoxic T-lymphocyte (CTL) response mediated by CD8(+) and perhaps CD4(+) CTLs. The mechanisms by which HIV-1 escapes from this CTL response are only beginning to be understood. However, it is already clear that the extreme genetic variability of the virus is a major contributing factor. Because of the well-known ability of altered peptide ligands (APL) to induce a T-cell receptor (TCR)-mediated anergic state in CD4(+) helper T cells, we investigated the effects of HIV-1 sequence variations on the proliferation and cytotoxic activation of a human CD4(+) CTL clone (Een217) specific for an epitope composed of amino acids 410 to 429 of HIV-1 gp120. We report that a natural variant of this epitope induced a functional anergic state rendering the T cells unable to respond to their antigenic ligand and preventing the proliferation and cytotoxic activation normally induced by the original antigenic peptide. Furthermore, the stimulation of Een217 cells with this APL generated altered TCR-proximal signaling events that have been associated with the induction of T-cell anergy in CD4(+) T cells. Importantly, the APL-induced anergic state of the Een217 T cells could be prevented by the addition of interleukin 2, which restored their ability to respond to their nominal antigen. Our data therefore suggest that HIV-1 variants can induce a state of anergy in HIV-specific CD4(+) CTLs. Such a mechanism may allow a viral variant to not only escape the CTL response but also facilitate the persistence of other viral strains that may otherwise be recognized and eliminated by HIV-specific CTLs.
Figures





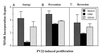
Similar articles
-
Brucella abortus conjugated with a peptide derived from the V3 loop of human immunodeficiency virus (HIV) type 1 induces HIV-specific cytotoxic T-cell responses in normal and in CD4+ cell-depleted BALB/c mice.J Virol. 1996 May;70(5):3084-92. doi: 10.1128/JVI.70.5.3084-3092.1996. J Virol. 1996. PMID: 8627787 Free PMC article.
-
Induction of a major histocompatibility complex class I-restricted cytotoxic T-lymphocyte response to a highly conserved region of human immunodeficiency virus type 1 (HIV-1) gp120 in seronegative humans immunized with a candidate HIV-1 vaccine.J Virol. 1994 May;68(5):3145-53. doi: 10.1128/JVI.68.5.3145-3153.1994. J Virol. 1994. PMID: 7908700 Free PMC article.
-
Recognition of a highly conserved region of human immunodeficiency virus type 1 gp120 by an HLA-Cw4-restricted cytotoxic T-lymphocyte clone.J Virol. 1993 Jan;67(1):438-45. doi: 10.1128/JVI.67.1.438-445.1993. J Virol. 1993. PMID: 7677956 Free PMC article.
-
HIV-1 proteins in infected cells determine the presentation of viral peptides by HLA class I and class II molecules and the nature of the cellular and humoral antiviral immune responses--a review.Virus Genes. 1994 Jul;8(3):249-70. doi: 10.1007/BF01704519. Virus Genes. 1994. PMID: 7975271 Review.
-
HIV-1 induced AIDS is an allergy and the allergen is the Shed gp120--a review, hypothesis, and implications.Virus Genes. 2004 Apr;28(3):319-31. doi: 10.1023/b:viru.0000025778.56507.61. Virus Genes. 2004. PMID: 15266113 Review.
Cited by
-
Natural epitope variants of the hepatitis C virus impair cytotoxic T lymphocyte activity.World J Gastroenterol. 2010 Apr 28;16(16):1953-69. doi: 10.3748/wjg.v16.i16.1953. World J Gastroenterol. 2010. PMID: 20419832 Free PMC article.
-
COVID-19 therapy with mesenchymal stromal cells (MSC) and convalescent plasma must consider exosome involvement: Do the exosomes in convalescent plasma antagonize the weak immune antibodies?J Extracell Vesicles. 2020 Oct;10(1):e12004. doi: 10.1002/jev2.12004. Epub 2020 Nov 14. J Extracell Vesicles. 2020. PMID: 33304473 Free PMC article. Review.
-
T cell recognition of weak ligands: roles of signaling, receptor number, and affinity.Immunol Res. 2011 May;50(1):39-48. doi: 10.1007/s12026-011-8204-3. Immunol Res. 2011. PMID: 21365321 Free PMC article. Review.
-
Suboptimal stimulation by weak agonist epitope variants does not drive dysfunction of HIV-1-specific cytotoxic T lymphocyte clones.AIDS. 2019 Aug 1;33(10):1565-1574. doi: 10.1097/QAD.0000000000002259. AIDS. 2019. PMID: 31306165 Free PMC article.
-
Interleukin 23 produced by myeloid dendritic cells contributes to T-cell dysfunction in HIV type 1 infection by inducing SOCS1 expression.J Infect Dis. 2015 Mar 1;211(5):755-68. doi: 10.1093/infdis/jiu523. Epub 2014 Sep 18. J Infect Dis. 2015. PMID: 25234720 Free PMC article.
References
-
- Bachmann M F, Speiser D E, Zakarian A, Ohashi P S. Inhibition of TCR triggering by a spectrum of altered peptide ligands suggests the mechanism for TCR antagonism. Eur J Immunol. 1998;28:3110–3119. - PubMed
-
- Borrow P, Lewicki H, Wei X, Horwitz M S, Peffer N, Meyers H, Nelson J A, Gairin J E, Hahn B H, Oldstone M B, Shaw G M. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. - PubMed
-
- Boussiotis V A, Freeman G J, Berezovskaya A, Barber D L, Nadler L M. Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated Rap1. Science. 1997;278:124–128. - PubMed
-
- Callahan K M, Fort M M, Obah E A, Reinherz E L, Siliciano R F. Genetic variability in HIV-1 gp120 affects interactions with HLA molecules and T cell receptor. J Immunol. 1990;144:3341–3346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

