Virus-induced abrogation of transplantation tolerance induced by donor-specific transfusion and anti-CD154 antibody
- PMID: 10666251
- PMCID: PMC111702
- DOI: 10.1128/jvi.74.5.2210-2218.2000
Virus-induced abrogation of transplantation tolerance induced by donor-specific transfusion and anti-CD154 antibody
Abstract
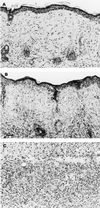
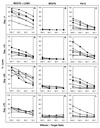
Treatment with a 2-week course of anti-CD154 antibody and a single transfusion of donor leukocytes (a donor-specific transfusion or DST) permits skin allografts to survive for >100 days in thymectomized mice. As clinical trials of this methodology in humans are contemplated, concern has been expressed that viral infection of graft recipients may disrupt tolerance to the allograft. We report that acute infection with lymphocytic choriomeningitis virus (LCMV) induced allograft rejection in mice treated with DST and anti-CD154 antibody if inoculated shortly after transplantation. Isografts resisted LCMV-induced rejection, and the interferon-inducing agent polyinosinic:polycytidylic acid did not induce allograft rejection, suggesting that the effect of LCMV is not simply a consequence of nonspecific inflammation. Administration of anti-CD8 antibody to engrafted mice delayed LCMV-induced allograft rejection. Pichinde virus also induced acute allograft rejection, but murine cytomegalovirus and vaccinia virus (VV) did not. Injection of LCMV approximately 50 days after tolerance induction and transplantation had minimal effect on subsequent allograft survival. Treatment with DST and anti-CD154 antibody did not interfere with clearance of LCMV, but a normally nonlethal high dose of VV during tolerance induction and transplantation killed graft recipients. We conclude that DST and anti-CD154 antibody induce a tolerant state that can be broken shortly after transplantation by certain viral infections. Clinical application of transplantation tolerance protocols may require patient isolation to facilitate the procedure and to protect recipients.
Figures


References
-
- Alexander-Miller M A, Burke K, Koszinowski U H, Hansen T H, Connolly J M. Alloreactive cytotoxic T lymphocytes generated in the presence of viral-derived peptides show exquisite peptide and MHC specificity. J Immunol. 1993;151:1–10. - PubMed
-
- Caldas C, Ambinder R. Epstein-Barr virus and bone marrow transplantation. Curr Opin Oncol. 1995;7:102–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

