Localization of cyclooxygenase-2 in human sporadic colorectal adenomas
- PMID: 10666384
- PMCID: PMC1850032
- DOI: 10.1016/S0002-9440(10)64759-1
Localization of cyclooxygenase-2 in human sporadic colorectal adenomas
Abstract
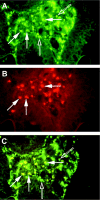
A putative target for the anti-colorectal cancer action of nonsteroidal anti-inflammatory drugs is the inducible isoform of cyclooxygenase (COX), COX-2. COX-2 is expressed within intestinal adenomas in murine polyposis models, but expression has been poorly characterized in human colorectal neoplasms. Therefore, we investigated the localization of the COX-2 protein in human sporadic colorectal adenomas. Immunohistochemistry for COX-2 and CD68 (a tissue macrophage marker) was performed on formalin-fixed, paraffin-embedded (n = 52) and frozen, acetone-fixed (n = 6) sections of human sporadic colorectal adenomas. Forty of 52 (77%) formalin-fixed adenomas expressed immunoreactive COX-2. COX-2 was localized to superficial interstitial macrophages in 39 cases (75%) and to deep interstitial macrophages in 9 cases (17%). COX-2 staining of dysplastic epithelial cells was observed in 15 cases (29%). A logistic regression analysis identified the adenoma site (P = 0.012) and histological type (P = 0.001) as independent predictors of superficial macrophage COX-2 expression. There was no relationship between the number of macrophages within an adenoma and macrophage COX-2 expression. These results indicate that COX-2 is expressed predominantly by interstitial macrophages within human sporadic colorectal adenomas. If COX-2 does indeed play a role in the early stages of colorectal carcinogenesis in man, these data suggest COX-2-mediated paracrine signaling between the macrophages and epithelial cells within adenomas.
Figures


References
-
- Levy GN: Prostaglandin H synthases, nonsteroidal anti-inflammatory drugs, and colon cancer. FASEB J 1997, 11:234-247 - PubMed
-
- Non-steroidal anti-inflammatory drugs. IARC Handbooks of Cancer Prevention, vol 1. Lyon, France, International Agency for Research on Cancer, 1997
-
- Shiff SJ, Rigas B: Nonsteroidal anti-inflammatory drugs and colorectal cancer: evolving concepts of their chemopreventive actions. Gastroenterology 1997, 113:1992-1998 - PubMed
-
- Herschman HR: Prostaglandin synthase 2. Biochim Biophys Acta 1996, 1299:125-140 - PubMed
-
- Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T: Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res 1995, 55:3785-3789 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

