Transactivation and growth suppression by the gut-enriched Krüppel-like factor (Krüppel-like factor 4) are dependent on acidic amino acid residues and protein-protein interaction
- PMID: 10666450
- PMCID: PMC102607
- DOI: 10.1093/nar/28.5.1106
Transactivation and growth suppression by the gut-enriched Krüppel-like factor (Krüppel-like factor 4) are dependent on acidic amino acid residues and protein-protein interaction
Abstract
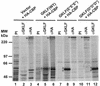
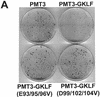
Gut-enriched Krüppel-like factor (GKLF or KLF4) is a pleiotropic (activating and repressive) transcription factor. This study characterizes the mechanisms of transactivation by GKLF. Using a GAL4 fusion assay, the activating domain of murine GKLF was localized to the 109 amino acid residues in the N-terminus. Site-directed mutagenesis showed that two adjacent clusters of acidic residues within this region are responsible for the activating effect. Transactivation by GKLF involves intermolecular interactions as demonstrated by the ability of wild-type, but not mutated, GKLF to compete with the N-terminal activation domain. In addition, wild-type adenovirus E1A, but not a mutated E1A that failed to bind p300/CBP, inhibited transactivation by the N-terminal 109 amino acids of GKLF, suggesting that p300/CBP are GKLF's interacting partners. A physical interaction between GKLF and CBP was demonstrated by glutathione- S -transferase pull-down and by in vivo co-immuno-precipitation experiments. We also showed that the two acidic amino acid clusters are essential for this interaction, since GKLF with mutations in these residues failed to co-immunoprecipitate with CBP. Importantly, the same mutations abrogated the ability of GKLF to suppress cell growth as determined by a colony suppression assay. These studies therefore provide plausible evidence for a structural and functional correlation between the transactivating and growth-suppressing effects of GKLF.
Figures








References
-
- Yang V.W. (1998) J. Nutr., 128, 2045–2051. - PubMed
-
- Johnson P.F. and McKnight,S.L. (1989) Annu. Rev. Biochem., 58, 799–839. - PubMed
-
- Mitchell P.J. and Tjian,R. (1989) Science, 245, 371–378. - PubMed
-
- Frankel A.D. and Kim,P.S. (1991) Cell, 65, 717–719. - PubMed
-
- McKnight S.L. and Yamamoto,K.R. (1992) Transcriptional Regulation. Cold Spring Harbor Laboratory, New York, NY.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

