CIZ, a zinc finger protein that interacts with p130(cas) and activates the expression of matrix metalloproteinases
- PMID: 10669742
- PMCID: PMC85348
- DOI: 10.1128/MCB.20.5.1649-1658.2000
CIZ, a zinc finger protein that interacts with p130(cas) and activates the expression of matrix metalloproteinases
Abstract
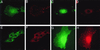
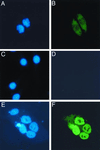
p130(cas) (Cas) is a docking protein that contains an SH3 domain and multiple tyrosine residues. p130(cas) is located at focal adhesions, is tyrosine phosphorylated in response to integrin stimulation, and is thought to transmit signals, via c-Crk and other proteins, for the remodeling of actin stress fibers and cell movement. In a search for the ligands of the SH3 domain of p130(cas) by far-Western screening, we cloned a novel protein named CIZ (for Cas-interacting zinc finger protein). CIZ consists of the following: a putative leucine zipper; a serine/threonine-rich region; a proline-rich sequence; five, six, or eight Krüppel-type C(2)H(2) zinc fingers; and the glutamine-alanine repeat. CIZ binds Cas in cells and is located in the nucleus and at focal adhesions. We showed that CIZ is a nucleocytoplasmic shuttling protein, by using the transient interspecies heterokaryon formation assay. In order to search for the targets of CIZ in nucleus, we determined the DNA binding consensus of CIZ as (G/C)AAAAA(A) by cyclic amplification and selection of targets analysis. The consensus-like sequences are found in several promoters of matrix metalloproteinases (MMPs), which are the enzymes used to degrade the extracellular matrix proteins. CIZ binds to a consensus-like sequence in the MMP-1 (collagenase) promoter. Overexpression of CIZ upregulates the transcriptions from MMP-1, MMP-3 (stromelysin), and MMP-7 (matrilysin) promoters, and this transactivation was enhanced in the presence of Cas. Furthermore, the stable overexpression of CIZ promoted the production of MMP-7 in culture medium. In summary, CIZ, a novel zinc finger protein, binds Cas, is a nucleocytoplasmic shuttling protein, and regulates the expression of MMPs.
Figures





References
-
- Astier A, Avraham H, Manie S N, Groopman J, Canty T, Avraham S, Freedman A S. The related adhesion focal tyrosine kinase is tyrosine-phosphorylated after beta1-integrin stimulation in B cells and binds to p130cas. J Biol Chem. 1997;272:228–232. - PubMed
-
- Borer R A, Lehner C F, Eppenberger H M, Nigg E A. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989;56:379–390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
