The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes
- PMID: 10669761
- PMCID: PMC85369
- DOI: 10.1128/MCB.20.5.1868-1876.2000
The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes
Abstract
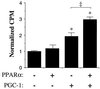
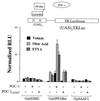
Peroxisome proliferator-activated receptor alpha (PPARalpha) plays a key role in the transcriptional control of genes encoding mitochondrial fatty acid beta-oxidation (FAO) enzymes. In this study we sought to determine whether the recently identified PPAR gamma coactivator 1 (PGC-1) is capable of coactivating PPARalpha in the transcriptional control of genes encoding FAO enzymes. Mammalian cell cotransfection experiments demonstrated that PGC-1 enhanced PPARalpha-mediated transcriptional activation of reporter plasmids containing PPARalpha target elements. PGC-1 also enhanced the transactivation activity of a PPARalpha-Gal4 DNA binding domain fusion protein. Retroviral vector-mediated expression studies performed in 3T3-L1 cells demonstrated that PPARalpha and PGC-1 cooperatively induced the expression of PPARalpha target genes and increased cellular palmitate oxidation rates. Glutathione S-transferase "pulldown" studies revealed that in contrast to the previously reported ligand-independent interaction with PPARgamma, PGC-1 binds PPARalpha in a ligand-influenced manner. Protein-protein interaction studies and mammalian cell hybrid experiments demonstrated that the PGC-1-PPARalpha interaction involves an LXXLL domain in PGC-1 and the PPARalpha AF2 region, consistent with the observed ligand influence. Last, the PGC-1 transactivation domain was mapped to within the NH(2)-terminal 120 amino acids of the PGC-1 molecule, a region distinct from the PPARalpha interacting domains. These results identify PGC-1 as a coactivator of PPARalpha in the transcriptional control of mitochondrial FAO capacity, define separable PPARalpha interaction and transactivation domains within the PGC-1 molecule, and demonstrate that certain features of the PPARalpha-PGC-1 interaction are distinct from that of PPARgamma-PGC-1.
Figures
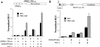



References
-
- Aoyama T, Peters J M, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez F J. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor α (PPARα) J Biol Chem. 1998;273:5678–5684. - PubMed
-
- Brandt J, Djouadi F, Kelly D P. Fatty acids activate transcription of the muscle carnitine palmitoyltransferase I gene in cardiac myocytes via the peroxisome proliferator-activated receptor α. J Biol Chem. 1998;273:23786–23793. - PubMed
-
- Cao Z, Umek R M, McKnight S L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5:1538–1552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
