Epidemiology of drug exposure and adverse drug reactions in two swiss departments of internal medicine
- PMID: 10671911
- PMCID: PMC2014906
- DOI: 10.1046/j.1365-2125.2000.00132.x
Epidemiology of drug exposure and adverse drug reactions in two swiss departments of internal medicine
Abstract
Aims: To explore drug exposure, frequency of adverse drug reactions (ADRs), types of ADRs, predisposing risk factors and ADR-related excess hospital stay in medical inpatients.
Methods: Structured data regarding patient characteristics, 'events' (symptoms, laboratory results), diagnoses (ICD10) and drug therapy were collected using a computer-supported data entry system and an interface for data retrieval from electronic patient records. ADR data were collected by 'event monitoring' to minimize possible bias by the drug monitor. The causality of each event was assessed in relation to disease(s) and drug therapy.
Results: The analysis included 4331 (100%) hospitalizations. The median observation period was 8 days. The median number of different drugs administered per patient and day was 6 and varied between 4 (Q1 ) and 9 (Q3 ) different drugs in 50% of all hospital days. In 41% of all hospitalizations at least one disease-unrelated event could be possibly attributed to drug therapy. Clinically relevant ADRs occurred in 11% of all hospitalizations. In 3.3% of all hospitalizations ADRs were the cause of hospital admission. The incidence of possibly ADR-related deaths was 1.4. Factors predisposing for clinically relevant ADRs were female gender and polypharmacy. ADR-related excess hospital stay accounted for 8. 6% of hospital days.
Conclusions: These data demonstrate the feasibility of the developed 'event monitoring' system for quantitative analysis of ADRs in medical inpatients. With increasing numbers of recorded patients the pharmacoepidemiological database provides a valuable tool to study specific questions regarding drug efficacy and safety in hospitalized patients.
Figures


 female.
female.
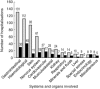
 ) and involved in ADR-related hospital admissions (
) and involved in ADR-related hospital admissions ( ) ADRs during cohort stay: Number of hospitalizations with at least one clinically relevant ADR involving the corresponding organ or system. Note that during some hospitalizations more than one clinically relevant ADR occurred and that some ADRs involved several organs or systems. ADR-related hospital admissions: Number of hospitalizations caused by an ADR involving mainly the corresponding organ or systems. Note that some ADRs involved several organs or systems.
) ADRs during cohort stay: Number of hospitalizations with at least one clinically relevant ADR involving the corresponding organ or system. Note that during some hospitalizations more than one clinically relevant ADR occurred and that some ADRs involved several organs or systems. ADR-related hospital admissions: Number of hospitalizations caused by an ADR involving mainly the corresponding organ or systems. Note that some ADRs involved several organs or systems.References
-
- Einarson TR. Drug-related hospital admissions. Ann Pharmacother. 1993;27:832–840. - PubMed
-
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–1205. - PubMed
-
- Jones JK, Kurata JH, Miwa L. Inpatient Databases. In: Strom BL, editor. Pharmacoepdiemiology. 2. Chichester: John Wiley & Sons; 1994. pp. 257–276.
-
- Lawson DH, Beard K. Intensive Hospital-Besed Cohort Studies. In: Strom BL, editor. Pharmacoepdiemiology. 2. Chichester: John Wiley & Sons; 1994. pp. 157–170.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

